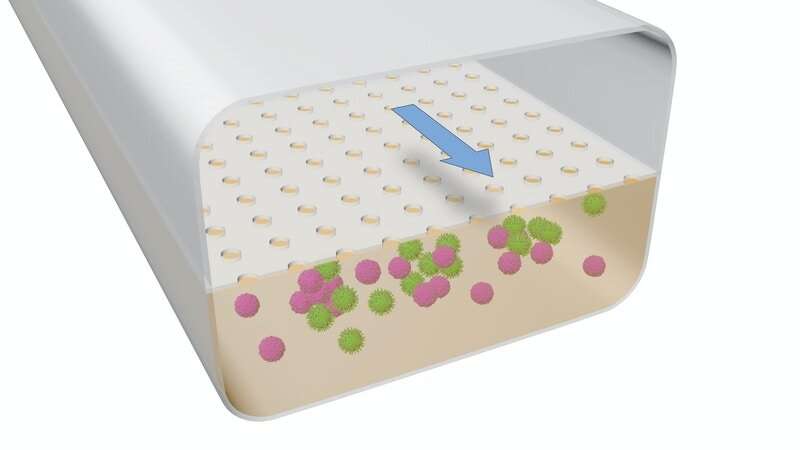
This illustration demonstrates the structure of the LF Chip: B cells and T cells were cultured together in the extracellular matrix (ECM)-lined lower channel, and were “fed” via the consistent flow of nutrient-containing medium through the upper channel. This flow is also what appears to have caused the spontaneous assembly of the cells into lymphoid follicles.
Credit: Wyss Institute at Harvard University
Lymphoid follicles formed in a microfluidic Organ Chip replicate human immune functions and vaccine responses in vitro
To quote veteran science writer Ed Yong’s simple yet extremely accurate words in The Atlantic, “The immune system is very complicated.” As the COVID-19 pandemic had made abundantly clear, science still doesn’t fully understand the sophisticated defense mechanisms that protect us from microbe invaders. Why do some people show no symptoms when infected with SARS-CoV-2 while others suffer from severe fevers and body aches? Why do some succumb to cytokine storms of the body’s own making? We still lack exact answers to these questions.
Today’s scientists, however, now have a new tool to help them tease out the immune system’s mysteries, thanks to a group of researchers at the Wyss Institute for Biologically Inspired Engineering at Harvard University. They cultured human B and T cells inside a microfluidic Organ Chip device and coaxed them to spontaneously form functional lymphoid follicles – structures that reside in lymph nodes and other parts of the human body which mediate immune responses. They consist of different chambers that harbor “naïve” B cells and T cells, which together initiate the cascade of events that leads to a full immune response when they are exposed to a specific antigen.
In addition to allowing researchers to probe the normal function of the immune system, these lymphoid follicle (LF) Chips can also be used to predict immune responses to various vaccines and help select the best performers, offering significant improvement over existing preclinical models like cells in a dish and non-human primates. The achievement is reported today in Advanced Science.
“Animals have been the gold-standard research models for developing and testing new vaccines, but their immune systems differ significantly from our own and do not accurately predict how humans will respond to them. Our LF Chip offers a way to model the complex choreography of human immune responses to infection and vaccination, and could significantly speed up the pace and quality of vaccine creation in the future,” said first author Girija Goyal, Ph.D., a Senior Staff Scientist at the Wyss Institute.
An accidental discovery
Like many great scientific discoveries, the LF Chip project is the result of serendipity in the lab. Goyal and other Wyss Institute scientists wanted to investigate how B and T cells circulating in the blood would change their behavior once they entered a tissue, so they obtained those cells from human blood samples and cultured them inside a microfluidic Organ Chip device to replicate the physical conditions they would experience when they encountered an organ.
When the cells were placed inside one of the two channels within the device, nothing remarkable happened — but when the researchers started the flow of culture medium through the other channel to feed the cells, they were surprised to see that the B and T cells started to spontaneously self-organize into 3D structures within the Organ Chip that appeared similar to “germinal centers” – structures within LFs where complex immune reactions take place. “It was so unexpected that we completely pivoted from the original experiment and focused on trying to figure out what they were,” said Goyal.
When the researchers started probing the mysterious structures that had formed inside the Organ Chip under flow conditions, they found that the cells were secreting a chemical called CXCL13. CXCL13 is a hallmark of LF formation, both within lymph nodes and in other parts of the body in response to chronic inflammation, such as in cancer and autoimmune conditions.
The team also found that B cells within the LFs that self-assembled on-chip also expressed an enzyme called activation-induced cytidine deaminase (AID), which is critical for activating B cells against specific antigens and is not present in B cells that are circulating in the blood. Neither CXCL13 nor AID were present in cells that were cultured in a standard 2D dish, suggesting that the scientists had indeed successfully created functional LFs from circulating blood cells.
In LFs in the human body, activated B cells mature and differentiate into multiple types of progeny cells including plasma cells, which secrete large amounts of antibodies against a specific pathogen. The team detected the presence of plasma cells in the LF Chips after they applied several stimuli used in the laboratory to activate B cells, such as the combination of the cytokine IL-4 and an anti-CD40 antibody, or dead bacteria. Remarkably, the plasma cells were concentrated in clusters within the LFs, as they would be in vivo.
“These findings were especially exciting because they confirmed that we had a functional model that could be used to unravel some of the complexities of the human immune system, including its responses to multiple types of pathogens,” said Pranav Prabhala, a Technician at the Wyss Institute and second author of the paper.
Predicting vaccine efficacy on-a-chip
Now that the scientists had a functional LF model that could initiate an immune response, they explored whether their LF Chip could be used to replicate and study the human immune system’s response to vaccines.
In the human body, vaccination induces special cells called dendritic cells to take up the injected pathogen and migrate to lymph nodes, where they present fragments of them on their surface. There, these antigen-presenting cells activate the B cells with the assistance of local T cells in the LF, causing the B cells to differentiate into plasma cells that produce antibodies against the pathogen. To replicate this process, the researchers added dendritic cells to LF Chips along with B and T cells from four separate human donors. They then inoculated the chips with a vaccine against the H5N1 strain of influenza along with an adjuvant called SWE that is known to boost immune responses to the vaccine.
Our LF Chip offers a way to model the complex choreography of human immune responses to infection and vaccination, and could significantly speed up the pace and quality of vaccine creation in the future.
LF Chips that received the vaccine and the adjuvant produced significantly more plasma cells and anti-influenza antibodies than B and T cells grown in 2D cultures or LF Chips that received the vaccine but not the adjuvant.
The team then repeated the experiment with cells from eight different donors, this time using the commercially available Fluzone? influenza vaccine, which protects against three different strains of the virus in humans. Once again, plasma cells and anti-influenza antibodies were present in significant numbers in the treated LF Chips. They also measured the levels of four cytokines in the vaccinated LF Chips that are known to be secreted by activated immune cells, and found that the levels of three of them (IFN-?, IL-10, and IL-2) were similar to those found in the serum of humans who had been vaccinated with Fluzone?.
The Wyss researchers are now using their LF Chips to test various vaccines and adjuvants in collaboration with pharmaceutical companies and the Gates Foundation.
“The flurry of vaccine development efforts sparked by the COVID-19 pandemic were impressive for their speed, but the increased demand suddenly made traditional animal models scarce resources. The LF Chip offers a cheaper, faster, and more predictive model for studying human immune responses to both infections and vaccines, and we hope it will streamline and improve vaccine development against many diseases in the future,” said corresponding author Donald Ingber, M.D., Ph.D., who is the Founding Director of the Wyss Institute as well as the Judah Folkman Professor of Vascular Biology at Harvard Medical School (HMS) and Boston Children’s Hospital, and Professor of Bioengineering at the Harvard John A. Paulson School of Engineering and Applied Sciences.
Original Article: The immune system is very complicated, but now, it’s on a chip
More from: Wyss Institute for Biologically Inspired Engineering
The Latest Updates from Bing News & Google News
Go deeper with Bing News on:
Organ Chip
- 'Britain's heaviest man' who weighed 50st dies from organ failure
‘ Britain’s heaviest man ’ has died only a week before his 34th birthday. Jason Holton, who weighed 50 stone, died last Saturday from organ failure. Six firefighters brought him to Royal Surrey County ...
- Avoiding meat may help reverse advanced liver cirrhosis
Advanced liver cirrhosis can push levels of ammonia in the blood to hazardous levels, but skipping meat at mealtime can help reverse that, new research shows.
- Organs on a Chip: Harvard Plans to Recreate the Human Body on Silicon
PCWorld helps you navigate the PC ecosystem to find the products you want and the advice you need to get the job done.
- The Crucial Role of Organ-on-a-Chip Technology in Understanding Mechanistic Toxicity
Organ-on-a-chip (OOC) technology offers a complementary solution to plug the gaps by providing a more physiologically relevant platform for drug safety assessment. High failure rates (30% due to ...
- CN Bio’s organ-on-a-chip technology boosted by £21m Series B raise
CN Bio is set to expand its PhysioMimix OOC (organ on a chip) technology and research services globally following the first close of a Series B investment round which has raised $21million.
Go deeper with Google Headlines on:
Organ Chip
[google_news title=”” keyword=”Organ Chip” num_posts=”5″ blurb_length=”0″ show_thumb=”left”]
Go deeper with Bing News on:
LF Chips
- Neuralink’s first human brain chip implant experienced a problem
If one was to just listen to Elon Musk, they might think the first Neuralink implant in a human was a flawless achievement."Successful 100 days with first human implant of @Neuralink," Musk posted to ...
- Lead Free Promise Project Promotes SB 514 For Early Testing
A lot of things happened in 1978; a ban on lead paint was not enough to fully reverse the negative effects of a toxic substance.
- Federal law delivers $28.6 million to Kansas for replacing lead pipes for drinking water
U.S. Environmental Protection Agency sends $28.6 million to replace lead water pipes in Kansas. The state ranks No. 3 in prevalence of lead water lines. The post Federal law delivers $28.6 million to ...
- Inquest called 15 years after man killed in jail over bag of chips
It’s been almost 15 years since Brantford-raised Jeff Munro was beaten to death in the Don Jail during an argument over a bag of potato chips. An inquest into his death has finally been announced.
- USDA Announces Publication of Cattle Tagging Program
The USDA is expected to publish new require electronic identification requirements ear tags for dairy, and some beef, cattle in May.
Go deeper with Google Headlines on:
LF Chips
[google_news title=”” keyword=”LF Chips” num_posts=”5″ blurb_length=”0″ show_thumb=”left”]