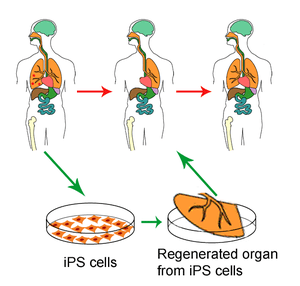
“PLURIPOTENT” is a long word that means “able to do many things”.
It is the technical term applied to stem cells that can generate many different sorts of bodily tissue, rather than just one sort, which is all that lesser stem cells can manage. But many researchers hope these cells will be pluripotent in other ways, too. Not only might they be used to make replacement tissues and organs for transplantation into those whose existing body parts no longer work properly (an approach known as regenerative medicine), they might also be used to produce pure cultures of cells for the early testing of drugs.
The culturing and use of human pluripotent stem cells is controversial because the natural source of such cells is embryos, which are destroyed by the process of extraction. But in 2007 two groups of researchers, one in Japan and the other in America, announced that they had worked out how to make adult skin cells behave like natural pluripotent cells, by adding four activated genes to them. The result is known as an induced pluripotent stem (iPS) cell.
On January 3rd AstraZeneca, a British drug company, said it would buy human heart muscle, blood vessels, nerve cells and liver cells made from iPS cells by Cellular Dynamics, a company founded by James Thomson (pictured). Dr Thomson, a biologist at the University of Wisconsin, is the man who led the team that, in 1998, isolated the first human embryonic stem cells and in 2007 published the American version of the iPS work.
AstraZeneca has two plans for its purchases. One is to use them to find molecules that encourage non-pluripotent stem cells to turn into mature tissue. Such molecules could act as drugs that coax damaged tissue to heal itself. The second is to use iPS cells to test drugs that have nothing to do with regenerative medicine.
Too often, a promising-looking drug fails late on in a clinical trial. Sometimes this is because it does not deliver the promised benefit. Sometimes, however, it works but its side effects prove more dangerous than the disease it is supposed to treat. The cost of failed trials is an important reason why drug research is so expensive. AstraZeneca therefore plans to use Cellular Dynamics’s cells to check the toxicity of potential medicines before they get inside a human being, in order to reduce the number of trials that fail for the second reason.
The Economist
The Latest Streaming News: Stem cells updated minute-by-minute
Bookmark this page and come back often
Latest NEWS
Latest VIDEO