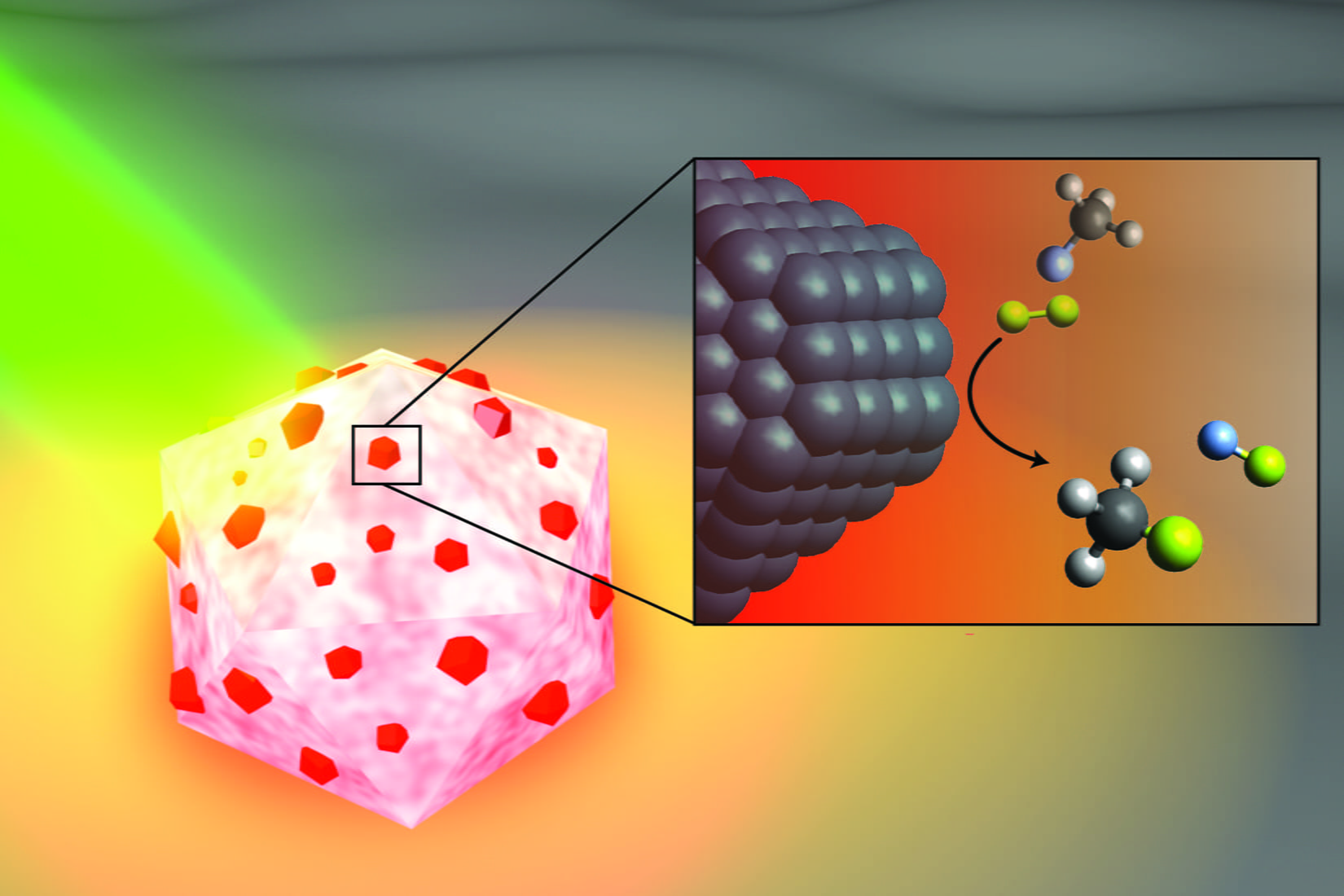
An artist’s illustration of the light-activated antenna-reactor catalyst Rice University engineers designed to break carbon-fluorine bonds in fluorocarbons. The aluminum portion of the particle (white and pink) captures energy from light (green), activating islands of palladium catalysts (red). In the inset, fluoromethane molecules (top) comprised of one carbon atom (black), three hydrogen atoms (grey) and one fluorine atom (light blue) react with deuterium (yellow) molecules near the palladium surface (black), cleaving the carbon-fluorine bond to produce deuterium fluoride (right) and monodeuterated methane (bottom). (Image courtesy of H. Robatjazi/Rice University)
Fluorocarbon bonds are no match for light-powered nanocatalyst
Rice U. lab unveils catalyst that can break problematic C-F bonds.
Rice University engineers have created a light-powered catalyst that can break the strong chemical bonds in fluorocarbons, a group of synthetic materials that includes persistent environmental pollutants.
In a study published this month in Nature Catalysis, Rice nanophotonics pioneer Naomi Halas and collaborators at the University of California, Santa Barbara (UCSB) and Princeton University showed that tiny spheres of aluminum dotted with specks of palladium could break carbon-fluorine (C-F) bonds via a catalytic process known as hydrodefluorination in which a fluorine atom is replaced by an atom of hydrogen.
The strength and stability of C-F bonds are behind some of the 20th century’s most recognizable chemical brands, including Teflon, Freon and Scotchgard. But the strength of those bonds can be problematic when fluorocarbons get into the air, soil and water. Chlorofluorocarbons, or CFCs, for example, were banned by international treaty in the 1980s after they were found to be destroying Earth’s protective ozone layer, and other fluorocarbons were on the list of “forever chemicals” targeted by a 2001 treaty.
“The hardest part about remediating any of the fluorine-containing compounds is breaking the C-F bond; it requires a lot of energy,” said Halas, an engineer and chemist whose Laboratory for Nanophotonics (LANP) specializes in creating and studying nanoparticles that interact with light.
Over the past five years, Halas and colleagues have pioneered methods for making “antenna-reactor” catalysts that spur or speed up chemical reactions. While catalysts are widely used in industry, they are typically used in energy-intensive processes that require high temperature, high pressure or both. For example, a mesh of catalytic material is inserted into a high-pressure vessel at a chemical plant, and natural gas or another fossil fuel is burned to heat the gas or liquid that’s flowed through the mesh. LANP’s antenna-reactors dramatically improve energy efficiency by capturing light energy and inserting it directly at the point of the catalytic reaction.
In the Nature Catalysis study, the energy-capturing antenna is an aluminum particle smaller than a living cell, and the reactors are islands of palladium scattered across the aluminum surface. The energy-saving feature of antenna-reactor catalysts is perhaps best illustrated by another of Halas’ previous successes: solar steam. In 2012, her team showed its energy-harvesting particles could instantly vaporize water molecules near their surface, meaning Halas and colleagues could make steam without boiling water. To drive home the point, they showed they could make steam from ice-cold water.
The antenna-reactor catalyst design allows Halas’ team to mix and match metals that are best suited for capturing light and catalyzing reactions in a particular context. The work is part of the green chemistry movement toward cleaner, more efficient chemical processes, and LANP has previously demonstrated catalysts for producing ethylene and syngas and for splitting ammonia to produce hydrogen fuel.
Study lead author Hossein Robatjazi, a Beckman Postdoctoral Fellow at UCSB who earned his Ph.D. from Rice in 2019, conducted the bulk of the research during his graduate studies in Halas’ lab. He said the project also shows the importance of interdisciplinary collaboration.
“I finished the experiments last year, but our experimental results had some interesting features, changes to the reaction kinetics under illumination, that raised an important but interesting question: What role does light play to promote the C-F breaking chemistry?” he said.
The answers came after Robatjazi arrived for his postdoctoral experience at UCSB. He was tasked with developing a microkinetics model, and a combination of insights from the model and from theoretical calculations performed by collaborators at Princeton helped explain the puzzling results.
“With this model, we used the perspective from surface science in traditional catalysis to uniquely link the experimental results to changes to the reaction pathway and reactivity under the light,” he said.
The demonstration experiments on fluoromethane could be just the beginning for the C-F breaking catalyst.
“This general reaction may be useful for remediating many other types of fluorinated molecules,” Halas said.
The Latest Updates from Bing News & Google News
Go deeper with Bing News on:
Fluorocarbon bonds
- Italy 10 Year Government Bond
U.S. 10 Year Treasury Note 0.0970 4.6690% ...
- Is it possible to short sell a bond?
Because bonds, like any other security, experience market fluctuations, traders may be eager to profit from a bet that the price of a bond will go lower. You can sell a bond short, but it can be ...
- The Best Bond Index Funds: Part 1
Part of the reason was that indexers began with stock funds, turning to bonds only later. Another was that active equity managers were the first to become discredited. By 2010, they were ...
- The Term “Bottled-in-Bond” Whiskey Might Not Mean What You Think It Does
Haara is referring to the Bottled-in-Bond Act of 1897, the nation’s first consumer protection law, which essentially protects people from buying or using shoddy products. In the case of whiskey, it ...
- Now Is Not The Time To Buy Bonds
The fundamental outlook for bonds remains questionable at best. The leading indicators of growth and inflation for 2024 suggest both dynamics could put upside pressure on yields. Sentiment toward ...
Go deeper with Google Headlines on:
Fluorocarbon bonds
[google_news title=”” keyword=”fluorocarbon bonds” num_posts=”5″ blurb_length=”0″ show_thumb=”left”]
Go deeper with Bing News on:
Light-powered nanocatalyst
- Quantum leap: Scientists make light waves ‘stand still’
Scientists have discovered a novel method to manipulate light waves, using nanophotonics, by deforming the two-dimensional photonic crystal that contains them.
- China: Researchers build high-resolution lidar with lowest-power laser
Researchers at the University of Science and Technology of China (USTC) have developed a compact and lightweight single-photon LiDAR system that can be deployed in the air to generate high-resolution ...
- Flat optics revolutionize quantum light sources for enhanced communication and sensing
The convergence of flat optics and quantum light sources represents a major step forward in the quest for compact, efficient, and versatile quantum technologies. By harnessing the power of ...
- How light can vaporize water without the need for heat
Researchers discovered that light can cause evaporation of water from a surface without the need for heat. This 'photomolecular effect' could be important for understanding climate change and for ...
- Revolutionizing Renewable Energy: Innovative Salt Battery Efficiently Harvests Osmotic Power
A new semipermeable membrane doubles the osmotic energy output in estuaries, showing potential for sustainable power generation. Estuaries — where freshwater rivers meet the salty sea — are great ...
Go deeper with Google Headlines on:
Light-powered nanocatalyst
[google_news title=”” keyword=”light-powered nanocatalyst” num_posts=”5″ blurb_length=”0″ show_thumb=”left”]