 A cathode that lasted five times longer than earlier designs
A cathode that lasted five times longer than earlier designs
When it comes to improving the performance of lithium-ion batteries, no part should be overlooked – not even the glue that binds materials together in the cathode, researchers at SLAC and Stanford have found.
Tweaking that material, which binds lithium sulfide and carbon particles together, created a cathode that lasted five times longer than earlier designs, according to a report published last month in Chemical Science. The research results are some of the earliest supported by the Department of Energy‘s Joint Center for Energy Storage Research.
“We were very impressed with how important this binder was in improving the lifetime of our experimental battery,” said Yi Cui, an associate professor at SLAC and Stanford who led the research.
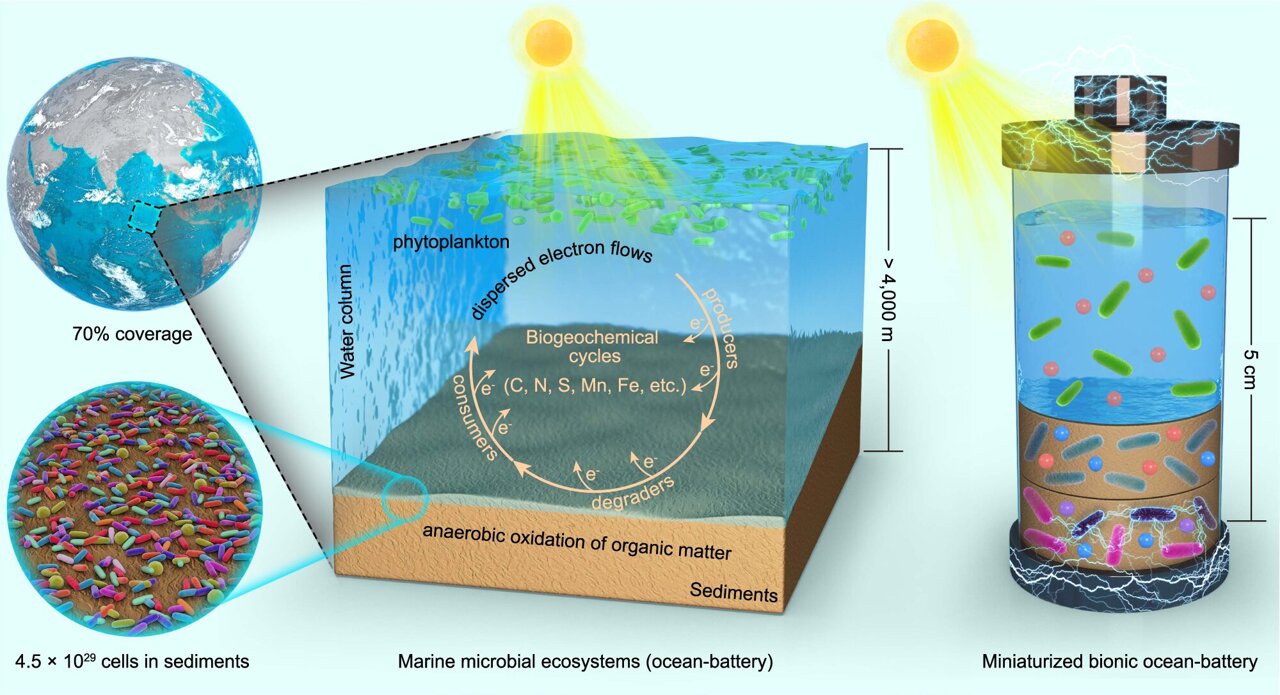
Researchers worldwide have been racing to improve lithium-ion batteries, which are one of the most promising technologies for powering increasingly popular devices such as mobile electronics and electric vehicles. In theory, using silicon and sulfur as the active elements in the batteries’ terminals, called the anode and cathode, could allow lithium-ion batteries to store up to five times more energy than today’s best versions. But finding specific forms and formulations of silicon and sulfur that will last for several thousand charge-discharge cycles during real-life use has been difficult.
Cui’s group was exploring how to create a better cathode by using lithium sulfide rather than sulfur. The lithium atoms it contains can provide the ions that shuttle between anode and cathode during the battery’s charge/discharge cycle; this in turn means the battery’s other electrode can be made from a non-lithium material, such as silicon. Unfortunately, lithium sulfide is also electrically insulating, which greatly reduces any battery’s performance. To overcome this, electrically conducting carbon particles can be mixed with the sulfide; a glue-like material – the binder – holds it all together.
Scientists in Cui’s group devised a new binder that is particularly well-suited for use with a lithium sulfide cathode – and that also binds strongly with intermediate polysulfide molecules that dissolve out of the cathode and diminish the battery’s storage capacity and useful lifetime.
The experimental battery using the new binder, known by the initials PVP, retained 94 percent of its original energy-storage capacity after 100 charge/discharge cycles, compared with 72 percent for cells using a conventionally-used binder, known as PVDF. After 500 cycles, the PVP battery still had 69 percent of its initial capacity.
The Latest Bing News on:
Battery Life
- Swiping to close iPhone apps actually hurts battery life, and Apple confirmed iton April 24, 2024 at 9:23 am
There’s always one person you know who opens Messages to send a text, then swipes to close the app right after. They say they’re saving … The post Swiping to close iPhone apps actually hurts battery ...
- These $23 Tozo earbuds have twice the battery life of AirPods — and they're over 60% offon April 24, 2024 at 7:34 am
Fans rave about how good these feel — and sound — in their ears: 'My favorite songs come to life in a way I've never experienced.' ...
- I changed these 10 iPhone settings and improved its battery life dramaticallyon April 22, 2024 at 5:57 am
If you're finding it hard to get through the day on a single charge with your iPhone, you might need to tweak some settings. Here's what to check.
- 10 iPhone settings I changed to dramatically improve battery lifeon April 19, 2024 at 3:10 pm
No matter how much you use your iPhone, you've almost certainly thought about how to maximize its battery life. After all, these phones -- particularly the smaller models-- can be more than just a ...
- How to improve battery life on the Samsung Galaxy Z Fold 5on April 16, 2024 at 12:12 pm
The Samsung Galaxy Z Fold 5 has a 4,400mAh battery, the same as its predecessor. When we tested the phone, it could reach more than seven hours of screen-on time while navigating with GPS and playing ...
- This iPhone setting will significantly improve your smartphone's battery life — prevent power-hungry apps without losing access to notificationson April 16, 2024 at 7:37 am
Make your iPhone's battery last longer. With our hectic daily lives, commutes, and unhealthy obsessions with doom scrolling, one of the most important aspects of any iPhone is its battery life.
- Marshall’s latest headphones get 100 hours of battery life and wireless chargingon April 16, 2024 at 7:27 am
When it comes time to do so, you’ll be able to drop the headphones on a wireless charger instead of using the included USB-C cable. Marshall also says it has preserved the Major’s sound signature ...
- Best phone battery life in 2024: The longest lasting smartphoneson April 15, 2024 at 7:30 pm
It's been a big year for long-lasting handsets, as new releases keep crashing onto our best phone battery life list. Two more phones have just crashed our list, joining the latest flagships from ...
- 6 Tips To Extend Your Fitbit Device's Battery Lifeon April 14, 2024 at 3:45 pm
If it seems like your Fitbit device's battery is draining faster than it should, here are six setting changes you can make for it to last longer.
The Latest Google Headlines on:
Battery Life
[google_news title=”” keyword=”Battery Life” num_posts=”10″ blurb_length=”0″ show_thumb=”left”]
The Latest Bing News on:
Cathode design
- Honda Canada To Grow EV Supply Chain With $15B Investmenton April 27, 2024 at 10:44 am
Over the last four years, automotive and battery makers announced more than $31 billion in investments in EV manufacturing across Canada.
- What’s that?…A fuel cell that harvests energy from…dirt?on April 26, 2024 at 7:10 am
A soil microbial fuel cell where microbes in dirt could power applications which are literally “in the field”.
- CATL's 1,000-km LFP EV battery super-charges at 1 km/secon April 25, 2024 at 8:54 pm
CATL made headlines around the globe last August when it presented the Shenxing battery, an LFP pack capable of adding 400 kilometers (249 miles) of range in a mere 10 minutes at the charger. Not a ...
- Sodium is safer and more abundant than lithiumon April 24, 2024 at 10:02 am
Sodium-ion batteries aren't new, but they've only started to gain traction in recent years. Compared to their lithium counterparts, the materials used in sodium batteries are ...
- Researchers Create an Iodine and Bromine-Based Multi-Electron Transfer Cathodeon April 24, 2024 at 9:57 am
In a new study published in Nature Energy , a research group led by Prof. Xianfeng Li from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) collaborated with ...
- Wildcat receives 100th patent for battery materials innovation and technologyon April 24, 2024 at 6:08 am
Battery materials pioneer Wildcat Discovery Technologies today announced it received its 100th patent, reinforcing its industry-leading innovation and advancing its strategy for U.S.-based cathode ...
- Engineering EV batteries for the 2030son April 22, 2024 at 9:02 pm
With SSBs being touted as a game-changer for EV battery technology, American SSB tech company QuantumScape has adopted a novel approach to battery design by doing away with the anode. Instead of an ...
- The Urwerk SpaceTime Blade: Straight From The Set Of ‘Star Wars’on April 22, 2024 at 4:45 am
The Urwerk SpaceTime Blade is a nixie clock reimagined for the Sci-Fi enthusiast, featuring 1.70-meter glass blade housing eight nixie tubes and displaying time with a futuristic twist ...
- Why some researchers think calcium is the future of batterieson April 16, 2024 at 9:55 am
Rechargeable versions of these low-cost power sources can be made today, but commercialization remains far down the road ...
- Elon Musk Re-Enters 'Wartime CEO' Mode? Tesla Chief Explains Necessity Of Massive Job Cuts Amid Report Of Critical Giga Texas Projects At Riskon April 15, 2024 at 8:29 pm
The cathode factory, led by Baglino (who departed), was intended ... Musk has previously described it as a revolutionary design “looking like the future.” Earlier this month, Reuters reported Tesla ...
The Latest Google Headlines on:
Cathode design
[google_news title=”” keyword=”cathode design” num_posts=”10″ blurb_length=”0″ show_thumb=”left”]