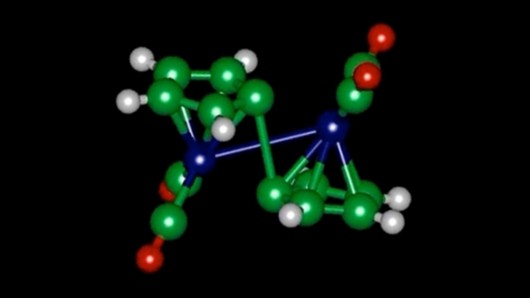
In figuring out how a molecule called fulvalene diruthenium works to store and release heat, researchers at MIT may have paved the way for a rechargeable battery that stores heat instead of electricity.
Although the molecule was discovered in 1996, ruthenium’s rarity and cost has ruled out it’s widespread use but the researchers say understanding the fundamental mechanism of how the molecule works should make it possible to find similar chemicals based on more abundant, less expensive materials.
When it absorbs sunlight, fulvalene diruthenium undergoes a structural change, putting it into a higher-energy state in which it can remain indefinitely. With the addition of a small amount of heat or a catalyst, it snaps back to its original shape, releasing heat in the process. But the tem found an intermediate step that plays a major role in the process whereby the molecule forms a semi-stable configuration partway between the two previously known states.
Jeffrey Grossman, the Carl Richard Soderberg Associate Professor of Power Engineering in the Department of Materials Science and Engineering says it is this unexpected two-step process that helps explain why the molecule is so stable and why the process is easily reversible and also why substituting other elements for ruthenium has not worked previously.
In effect, explained Grossman, this process makes it possible to produce a “rechargeable heat battery” that can repeatedly store and release heat gathered from sunlight or other sources. In principle, Grossman said, a fuel made from fulvalene diruthenium, when its stored heat is released, “can get as hot as 200 degrees C (392 F), plenty hot enough to heat your home, or even to run an engine to produce electricity.”
The Latest Streaming News: rechargeable heat battery updated minute-by-minute







