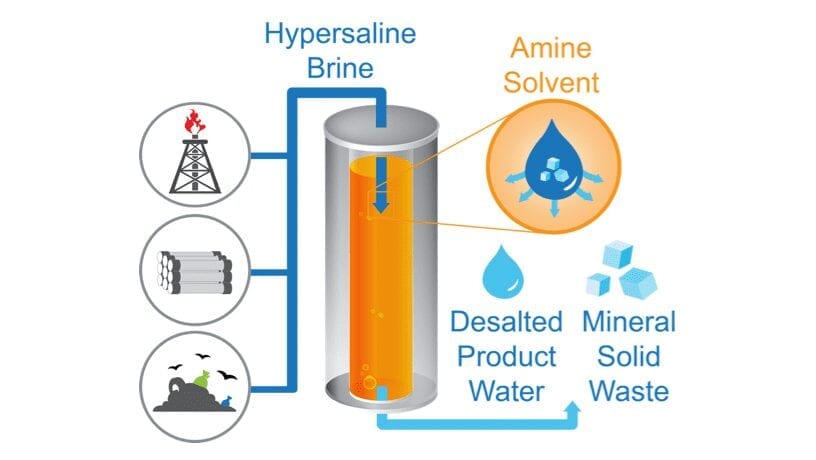
Illustration of the TSSE process, a pioneering desalination approach for hypersaline brines that could transform global water management
Water security is becoming an urgent global challenge. Hundreds of millions of people already live in water-scarce regions, and the UN projects that by 2030 about half the world’s population will be living in highly water-stressed areas. This will be a crisis even for developed countries like the U.S., where water managers in 40 states expect freshwater shortages within the next 10 years. As the global population and GDP grow, so will the demand for freshwater. And, with the continuing rise of global temperatures, water shortages will only get worse.
Desalination processes are increasingly being relied upon to augment water supplies. In fact, global desalination capacity is projected to double between 2016 and 2030. But these processes are expensive and can be harmful to the environment. The ultrahigh salinity brines that are the byproduct of desalination can be several times that of seawater salinity and its management options are especially challenging for inland desalination facilities such as those in Arizona, California, Florida, and Texas.
Over the past year, Columbia Engineering researchers have been refining their unconventional desalination approach for hypersaline brines—temperature swing solvent extraction (TSSE)—that shows great promise for widespread use. TSSE is radically different from conventional methods because it is a solvent-extraction-based technique that does not use membranes and is not based on evaporative phase-change: it is effective, efficient, scalable, and sustainably powered. In a new paper, published online June 23 in Environmental Science & Technology, the team reports that their method has enabled them to attain energy-efficient zero-liquid discharge (ZLD) of ultrahigh salinity brines—the first demonstration of TSSE for ZLD desalination of hypersaline brines.
“Zero-liquid discharge is the last frontier of desalination,” says Ngai Yin Yip, an assistant professor of earth and environmental engineering who led the study. “Evaporating and condensing the water is the current practice for ZLD but it’s very energy intensive and prohibitively costly. We were able to achieve ZLD without boiling the water off—this is a major advance for desalinating the ultrahigh salinity brines that demonstrates how our TSSE technique can be a transformative technology for the global water industry.”
Yip’s TSSE process begins with mixing a low-polarity solvent with the high salinity brine. At low temperatures (the team used 5 °C), the TSSE solvent extracts water from the brine but not salts (which are present in the brine as ions). By controlling the ratio of solvent to brine, the team can extract all the water from the brine into the solvent to induce the precipitation of salts—after all the water is “sucked” into the solvent, the salts form solid crystals and fall to the bottom, which can then be easily sieved out.
Desalination processes are increasingly being relied upon to augment water supplies. In fact, global desalination capacity is projected to double between 2016 and 2030. But these processes are expensive and can be harmful to the environment. The ultrahigh salinity brines that are the byproduct of desalination can be several times that of seawater salinity and its management options are especially challenging for inland desalination facilities such as those in Arizona, California, Florida, and Texas.
Over the past year, Columbia Engineering researchers have been refining their unconventional desalination approach for hypersaline brines—temperature swing solvent extraction (TSSE)—that shows great promise for widespread use. TSSE is radically different from conventional methods because it is a solvent-extraction-based technique that does not use membranes and is not based on evaporative phase-change: it is effective, efficient, scalable, and sustainably powered. In a new paper, published online June 23 in Environmental Science & Technology, the team reports that their method has enabled them to attain energy-efficient zero-liquid discharge (ZLD) of ultrahigh salinity brines—the first demonstration of TSSE for ZLD desalination of hypersaline brines.
“Zero-liquid discharge is the last frontier of desalination,” says Ngai Yin Yip, an assistant professor of earth and environmental engineering who led the study. “Evaporating and condensing the water is the current practice for ZLD but it’s very energy intensive and prohibitively costly. We were able to achieve ZLD without boiling the water off—this is a major advance for desalinating the ultrahigh salinity brines that demonstrates how our TSSE technique can be a transformative technology for the global water industry.”
Yip’s TSSE process begins with mixing a low-polarity solvent with the high salinity brine. At low temperatures (the team used 5 °C), the TSSE solvent extracts water from the brine but not salts (which are present in the brine as ions). By controlling the ratio of solvent to brine, the team can extract all the water from the brine into the solvent to induce the precipitation of salts—after all the water is “sucked” into the solvent, the salts form solid crystals and fall to the bottom, which can then be easily sieved out.
The Latest Updates from Bing News & Google News
Go deeper with Bing News on:
Global water management
- Central Highlands Water taps Infor for digital transformation
The new platform replaces five core legacy on-premise systems, including Infor Public Sector, and covers a wide range of business processes, from financial accounting, budgeting and planning, asset ...
- El Nino, water management issues blamed for snarling Panama Canal
The El Nino climate phenomenon, not climate change, drove lower rainfall last year that reduced the Panama Canal's water levels and contributed to shipping restrictions that disrupted global trade, a ...
- Minister emphasizes RI's commitment on sustainable water management
Coordinating Minister for Maritime Affairs and Investment Luhut Binsar Pandjaitan emphasized the government's commitment to realizing integrated and ...
- Researchers, water managers gather to discuss challenges facing Truckee River watershed
Conference attendees, in addition to receiving information from University researchers, expressed an interest in sharing their concerns about water management ... Climate change — long-terms shifts in ...
- Global Water Resources Declares Monthly Dividend
Global Water Resources, Inc. (NASDAQ: GWRS), a pure-play water resource management company, has declared, under its dividend policy a monthly cash dividend in the amount of $0.02508 per common share ...
Go deeper with Google Headlines on:
Global water management
[google_news title=”” keyword=”global water management” num_posts=”5″ blurb_length=”0″ show_thumb=”left”]
Go deeper with Bing News on:
Desalination
- Wave of innovation aims to make desalination sustainable
In the grip of a three-year drought, the Spanish city of Barcelona announced last month that it would have to start supplying its 1.6 million inhabitants with water brought by boat from a desalination ...
- LG Chem pledges $86.6 million for water desalination device plant in Saudi Arabia
LG Chem will invest up to 120 billion won ($86.6 million) jointly with Alkhorayef Group to build its first overseas water desalination device plant in Saudi Arabia by 2026.
- Novel PV-driven desalination tech achieves lower levelized cost of water
Scientists led by the Massachusetts Institute of Technology (MIT) have designed a new PV-powered desalination system based on the time-variant electrodialysis reversal (EDR) technology. The proposed ...
- ACCIONA PRESENTS IT FIRST TALKS ABOUT REVERSE OSMOSIS DESALINATION
ACCIONA, a global company and a leader in the provision of regenerative solutions for a decarbonized economy, held its first ...
- Water desalination plays important role in global water challenges
Desalination of saltwater, once seen as a distant solution to the global water crisis, is increasingly becoming a practical option, particularly in r ...
Go deeper with Google Headlines on:
Desalination
[google_news title=”” keyword=”desalination ” num_posts=”5″ blurb_length=”0″ show_thumb=”left”]