UC Berkeley chemists tested their chemical process for breaking down polyethylene plastic on several types of post-consumer PE: four-pack tops, milk and shampoo bottles and plastic packaging. The yield of propylene from each is shown at the bottom. With improvements to the process, it could make a dent in the PE waste stream and provide a product in high-demand by the plastics industry without using more fossil fuels.
(Photo credit: John Hartwig lab, UC Berkeley)
Polyethylene plastics — in particular, the ubiquitous plastic bag that blights the landscape — are notoriously hard to recycle. They’re sturdy and difficult to break down, and if they’re recycled at all, they’re melted into a polymer stew useful mostly for decking and other low-value products.
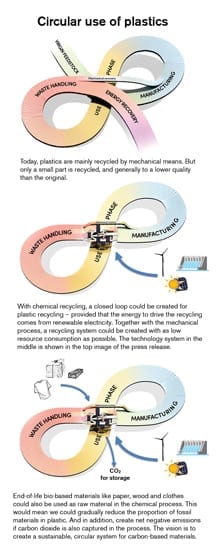
But a new process developed at the University of California, Berkeley, and Lawrence Berkeley National Laboratory (Berkeley Lab) could change all that. The process uses catalysts to break the long polyethylene (PE) polymers into uniform chunks — the three-carbon molecule propylene — that are the feedstocks for making other types of high-value plastic, such as polypropylene.
The process, admittedly in the early stages of development, would turn a waste product — not only plastic bags and packaging, but all types of PE plastic bottles — into a major product in high demand. Previous methods to break the chains of polyethylene required high temperatures and gave mixtures of components in much lower demand. The new process could not only lower the need for fossil fuel production of propylene, often called propene, but also help fill a currently unmet need by the plastics industry for more propylene.
“To the extent they get recycled, a lot of polyethylene plastics get turned into low-grade materials. You can’t take a plastic bag and then make another plastic bag with the same properties out of it,” said John Hartwig, UC Berkeley’s Henry Rapoport Chair in Organic Chemistry. “But if you can take that polymer bag back to its monomers, break it down into small pieces and repolymerize it, then instead of pulling more carbon out of the ground, you use that as your carbon source to make other things — for example, polypropylene. We would use less shale gas for that purpose, or for the other uses of propene, and to fill the so-called propylene gap.”
Polyethylene plastics make up about one-third of the entire plastics market worldwide, with more than 100 million tons produced yearly from fossil fuels, including natural gas obtained by hydraulic fracturing, often called shale gas.
Despite recycling programs — recyclable PE products are designated with plastic numbers 2 and 4 — only about 14% of all polyethylene plastic products are recycled. Because of their stability, polyethylene polymers are difficult to break down into their component parts, or depolymerize, so most of the recycling involves melting it and molding it into other products, like yard furniture, or burning it as fuel.
Depolymerizing polyethylene and turning into proplylene is a way of upcycling — that is, producing higher-value products from essentially zero-value waste, while reducing the use of fossil fuels.
Hartwig and his colleagues will publish the details of their new catalytic process this week in the journal Science.
Two types of catalysts
Hartwig specializes in using metal catalysts to insert unusual and reactive bonds into hydrocarbon chains, most of which are petroleum based. Novel chemical groups can then be added at these reactive bonds to form new materials. The hydrocarbon polyethylene, which typically occurs as a polymer chain of perhaps 1,000 ethylene molecules — each ethylene is composed of two carbon and four hydrogen atoms — provided his team with a challenge because of its general non-reactivity.
Original Article: Process converts polyethylene bags, plastics to polymer building blocks
More from: Lawrence Berkeley National Laboratory | University of California Berkeley
The Latest Updates from Bing News & Google News
Go deeper with Bing News on:
Polyethylene plastics recycling
- Plastic Pail Earns Sustainable Stripes
Milacron and partners perfect the injection molding of a precise layer of recycled content — mechanical or advanced processes — to sustainably boost the eco-footprint of 5-gal plastic pails.
- New Yorkers are worried about the waste crisis but don’t see a plastic ban as fix
Despite the essential role plastics play in daily life, with about 70% of New Yorkers finding plastics indispensable, there is a strong consensus against eliminating plastic use. A substantial number ...
- deSter Partners With Eastman To Eliminate Single-Use Plastic Waste in the Airline Industry
Ster, a leading global provider of innovative service ware and food packaging concepts for the travel and food service industries, and Eastman, a global specialty materials company, are collaborating ...
- How Politics And Litigation Impede Innovation In Plastics Recycling
Plastics recycling has long been one of society's most intractable problems. A planned new set of court actions seems set to only delay real solutions.
- Recycle ag plastics in West Baraboo
The Sauk County Land Resources & Environment Department will hold a free agricultural plastics recycling drop-off event from 10 a.m. to noon on Wednesday at the Sauk County Highway Shop, 620 Linn St., ...
Go deeper with Bing News on:
Polyethylene upcycling
- Ames National Laboratory team transforms plastic waste into fuel
When Yi-Yu Wang pictures the future, she hopes for a day when someone can just pop an empty water bottle in their car’s tank when running low on gas in order to deliver the energy it needs to keep ...
- Mycocycle uses mushrooms to upcycle old tires and construction waste
Usually, when something starts to rot, it gets pitched in the trash. But Joanne Rodriguez wants to turn the concept of rot on its head by growing fungus ...
- Scientists explore nature’s promise in combating plastic waste
By Claire Asher Plastic is a remarkably versatile and durable material, which has made it indispensable in almost every area of modern life. But these same properties, amplified by our ...
- Cold sintering may rescue plastic, ceramics, battery components from landfills
Recycling does not necessarily prevent an item from eventually ending up in a landfill, according to Enrique Gomez, interim associate dean for equity and inclusion and professor of chemical ...
- Recycle, Upcycle, Repurpose: Finding Ways To Reduce Landfill Waste
According to the United States Environmental Protection Agency (EPA), the U.S. generated about 292 million tons of municipal solid waste in 2018; the latest year figures are available. People ...