New research at MIT could dramatically improve the efficiency of fuel cells, which are considered a promising alternative to batteries for powering everything from electronic devices to cars and homes.
Fuel cells make electricity by combining hydrogen, or hydrocarbon fuels, with oxygen. But the most efficient types, called solid oxide fuel cells (SOFC), have drawbacks that have limited their usefulness — including operating temperatures above 700 degrees Celsius (roughly 1300 degrees Fahrenheit). Now, MIT researchers have unraveled the properties of a promising alternative material structure for a key component of these devices.
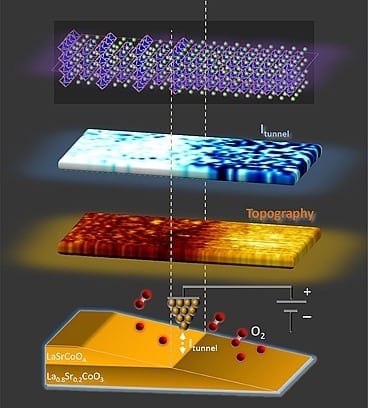
The new structure, a “superlattice” of two compounds interleaved at a tiny scale, could serve as one of the two electrodes in the fuel cell. The complex material, discovered about six years ago and known as LSC113/214, is composed of two oxides of the elements lanthanum, strontium and cobalt. While one of the oxides was already known as an especially good material for such electrodes, the combination of the two is far more potent in promoting oxygen reduction than either oxide alone.
The interfaces between these two oxides were thought to be the key. But until now, no one had been able to observe the LSC113/214 interface properties in operation, at sufficiently high resolution, to figure out why it worked so well.
Oxygen reduction is one of two main reactions in a fuel cell, and the one that has limited their overall performance — so finding improved materials for that reaction could be a key advance for fuel cells, the researchers say. The new findings are published in the journal Advanced Energy Materials in a paper co-authored by graduate student Yan Chen, professors Harry Tuller and Bilge Yildiz, and three other researchers at MIT.
Yildiz, an associate professor of nuclear science and engineering, says LSC113/214 has been “a singular example” of a material with extremely high reactivity to oxygen reduction; the new results explaining why it works so well could lead to further optimization or the discovery of other materials that might perform even better.
The best of both
The key to the material’s performance, she explains, is the marriage of complementary qualities from its two constituents. One of the oxides allows superior conduction and transfer of electrons, while the other excels at holding onto oxygen atoms; to perform well as a fuel cell’s cathode — one of its two electrodes — a material needs to have both qualities.
The close proximity of the two materials in this superlattice causes them to “borrow” one another’s attributes, the MIT team found. The result is a material whose reactivity exceeds that of the best materials currently used in fuel cells, Yildiz says: “It’s the best of the two worlds.”
Now that the MIT team has analyzed LSC113/214, it may be possible to discover even better materials by conducting systematic searches, Yildiz says; the team is now working on that. “If we can crack this problem, then we can make great strides in improving the performance,” adds Tuller, a professor of ceramics and electronic materials in MIT’s Department of Materials Science and Engineering.
The Latest Bing News on:
Fuel cells
- Varanasi pilot location for deployment of green hydrogen fuel cell inland vessels on NW-Ion April 27, 2024 at 3:13 pm
New Delhi, Varanasi has been selected as the pilot location for deployment of green hydrogen fuel cell inland vessels on National Waterways-I, aligning with the government's Harit Nauka guidelines, an ...
- California Welcomes First Big-Rig Hydrogen Fuel Station in U.S.on April 26, 2024 at 9:08 am
The country’s first commercial hydrogen fuel station for big-rig trucks is up and running at the Port of Oakland, a step toward what hydrogen proponents see as a clean new future for long-haul ...
- What’s that?…A fuel cell that harvests energy from…dirt?on April 26, 2024 at 7:10 am
A soil microbial fuel cell where microbes in dirt could power applications which are literally “in the field”.
- LaFeO3 Perovskite Fuel Cell Technology Boosts SRMAP's Clean Energy Missionon April 26, 2024 at 6:45 am
The significance of this invention cannot be understated in an era where the reliance on clean and efficient energy conversion methods is paramount ...
- RICE develops new underwater robot with a buoyancy control system using fuel cellson April 26, 2024 at 4:31 am
Rice University students have made a breakthrough in underwater robotics by creating a remotely operated vehicle (ROV) that utilizes water-splitting fuel cells for buoyancy control. This innovative ...
- Revolutionizing Clean Energy: SRMAP’s Breakthrough in Fuel Cell Technology with LaFeO3 Perovskiteon April 25, 2024 at 11:47 pm
In the quest for sustainable energy solutions to meet the soaring global energy demands, an innovative breakthrough has emerged from the laboratories of SRM University-AP, situated in the picturesque ...
- GILLIG to develop hydrogen fuel cell powered buson April 25, 2024 at 9:05 am
GILLIG is collaborating with BAE Systems and Ballard Power Systems on the development of the hydrogen fuel cell bus.
- This legendary brand cracks the code: here’s the most powerful hydrogen fuel cellon April 25, 2024 at 7:29 am
Legendary brand has created the first lightweight hydrogen fuel cell: the most powerful, but the smallest ever seen in history ...
- The first big-rig hydrogen fuel station in the U.S. opens in Californiaon April 24, 2024 at 3:00 am
The Port of Oakland is home to the United States' first commercial hydrogen fuel station for big-rig trucks. It's a step on the road to cleaner trucking.
- New Hydrogen fuel cell makes zero-emission flights a possibilityon April 18, 2024 at 2:47 am
Zero Avia has made a groundbreaking advancement in aviation technology with the development of their hydrogen fuel cell system. This innovation represents ...
The Latest Google Headlines on:
Fuel cells
[google_news title=”” keyword=”fuel cells” num_posts=”10″ blurb_length=”0″ show_thumb=”left”]
The Latest Bing News on:
Solid oxide fuel cells
- Is Bloom Energy Stock a Buy?on April 27, 2024 at 12:12 am
The Bloom Energy Server uses its proprietary solid oxide technology to convert fuel (natural gas, biogas, hydrogen) through an electrochemical process without combustion. These servers can be ...
- Mitsubishi Heavy Industries begins testing solid oxide electrolyzer cell for hydrogen productionon April 26, 2024 at 6:01 pm
The SOEC test module includes multiple cartridges of 500 cell stacks bundled together. MHI said results will be used to support higher output and capacity.
- Korean researchers build 8 kW solid oxide electrolysis cell that can produce 5.7 kg of hydrogen per dayon April 26, 2024 at 3:23 am
The Korea Institute of Energy Research has developed a solid oxide electrolysis cell stack that uses a special kind of separator plate to ensure proper flow of hydrogen and oxigen after water ...
- 3 Hydrogen Stocks That Could Be Multibaggers in the Making: April Editionon April 25, 2024 at 3:15 pm
InvestorPlace - Stock Market News, Stock Advice & Trading Tips There are some multibagger hydrogen stocks that investors should have on their ...
- Plasma treatment enhances electrode material for fuel cells in industry, homes and vehicleson April 22, 2024 at 8:52 am
Researchers from Skoltech and their colleagues have improved the properties of a carbon-based electrode material by exposing it to air plasma. Such treatment turned out to enhance electrode ...
- Manufacturer Gets $75 Million in Tax Credits to Boost Fuel Cell Productionon April 19, 2024 at 4:07 am
FREMONT, CA—Bloom Energy Inc. has been awarded up to $75 million in tax credits from the federal government to expand domestic manufacturing of solid oxide fuel cells at its assembly plant here.
- Market size of solid oxide fuel cells APAC 2020-2028on April 17, 2024 at 5:00 pm
Market size of solid oxide fuel cells (SOFC) in the Asia-Pacific region from 2020 to 2023, with a forecast for 2028 (in million U.S. dollars) Characteristic Market size in million U.S. dollars ...
- HyAxiom and Doosan Fuel Cell Are World's First to Pass Solid Oxide Fuel Cell Components Environmental Testing for Maritime Applicationon March 24, 2024 at 5:00 pm
and Doosan Fuel Cell Co., Ltd. (DFCC) announced today their Solid Oxide Fuel Cell (SOFC) cell stack has passed a critical environmental test by Det Norske Veritas (DNV), the classification society ...
- Chapter 1: Solid Oxide Fuel Cellson February 21, 2018 at 8:54 am
A Solid Oxide Fuel Cell (SOFC) is typically composed of two porous electrodes, interposed between an electrolyte made of a particular solid oxide ceramic material. The system originates from the work ...
- Turnkey Test Kits for Planar Solid Oxide Fuel Cells (SOFCs)on April 26, 2016 at 6:22 am
The kits are designed to provide a very effective solution to test individual planar solid oxide fuel cells. All of the kits are equipped with the appropriate items required to operate five tests, ...
The Latest Google Headlines on:
Solid oxide fuel cells
[google_news title=”” keyword=”solid oxide fuel cells” num_posts=”10″ blurb_length=”0″ show_thumb=”left”]