While the human race will always leave its carbon footprint on the Earth, it must continue to find ways to lessen the impact of its fossil fuel consumption.
“Carbon capture” technologies – chemically trapping carbon dioxide before it is released into the atmosphere – is one approach. In a recent study, Cornell University researchers disclose a novel method for capturing the greenhouse gas and converting it to a useful product – while producing electrical energy.
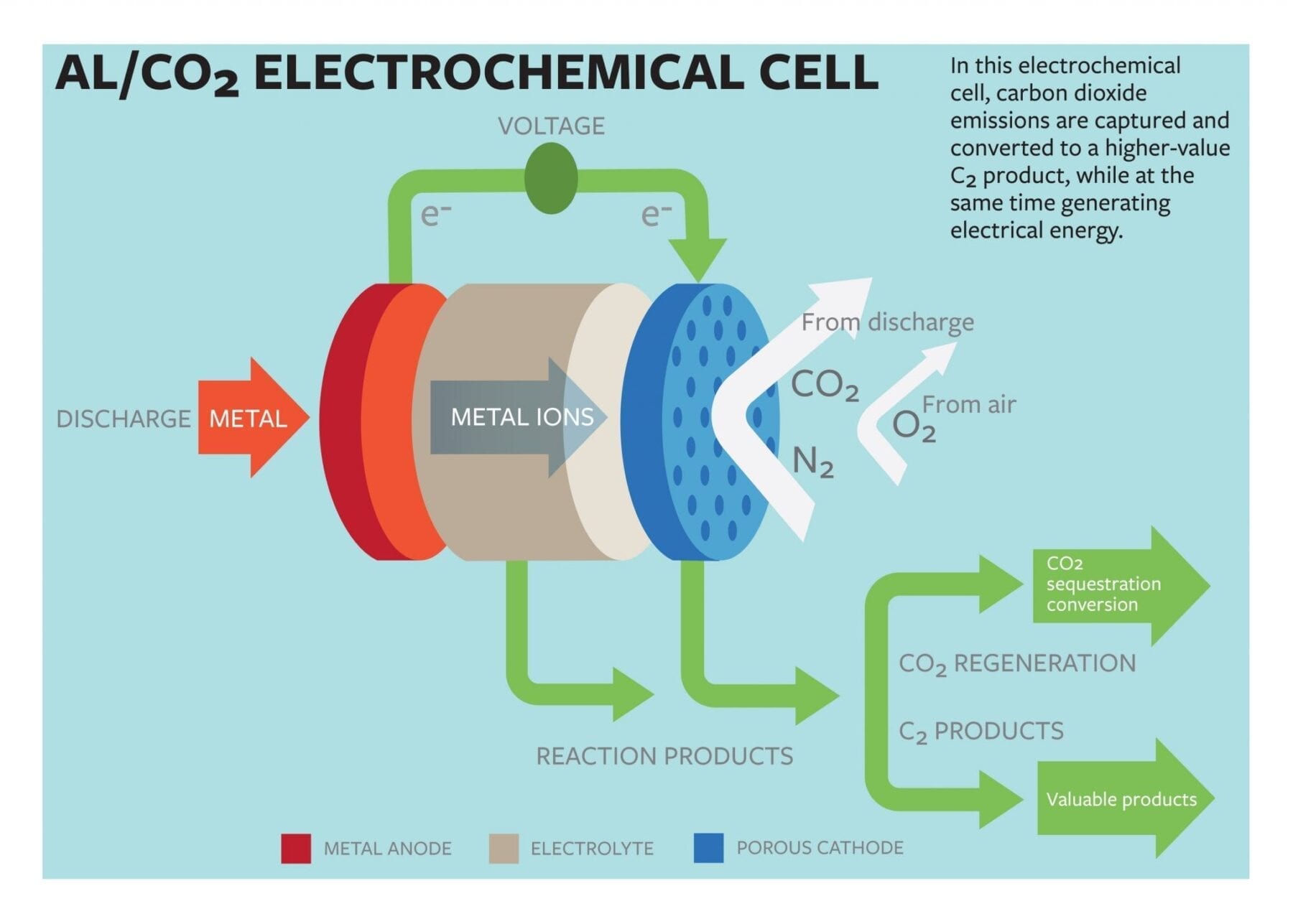
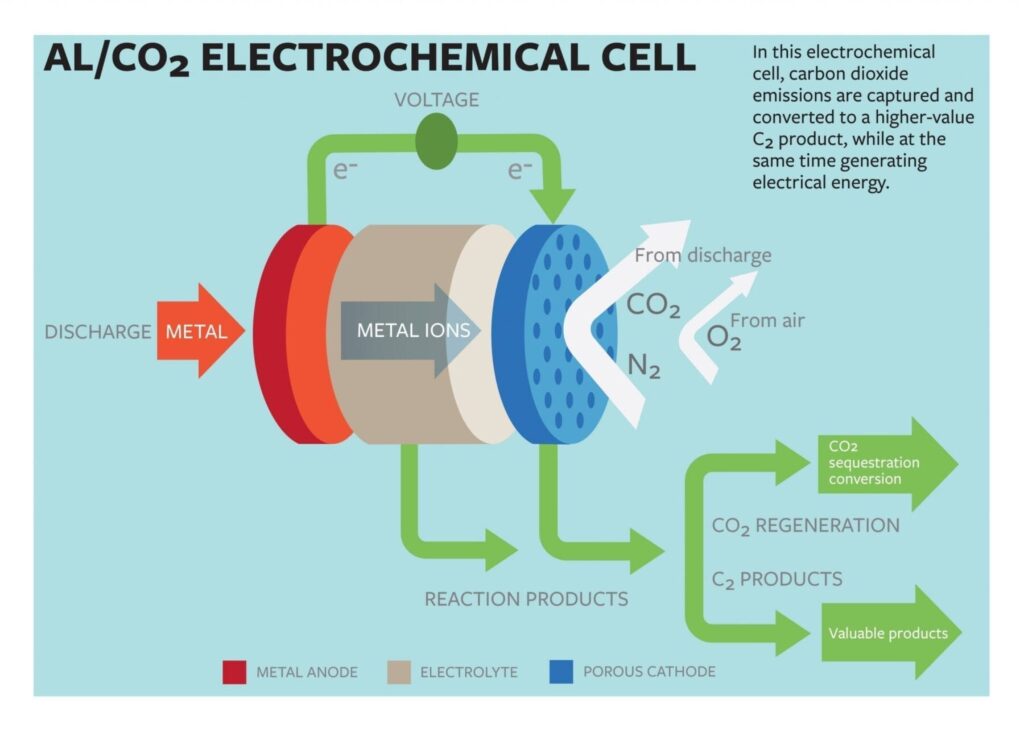
Lynden Archer, the James A. Friend Family Distinguished Professor of Engineering, and doctoral student Wajdi Al Sadat have developed an oxygen-assisted aluminum/carbon dioxide power cell that uses electrochemical reactions to both sequester the carbon dioxide and produce electricity.
Their paper, “The O2-assisted Al/CO2 electrochemical cell: A system for CO2capture/conversion and electric power generation,” was published July 20 in Science Advances.
The group’s proposed cell would use aluminum as the anode and mixed streams of carbon dioxide and oxygen as the active ingredients of the cathode. The electrochemical reactions between the anode and the cathode would sequester the carbon dioxide into carbon-rich compounds while also producing electricity and a valuable oxalate as a byproduct.
In most current carbon-capture models, the carbon is captured in fluids or solids, which are then heated or depressurized to release the carbon dioxide. The concentrated gas must then be compressed and transported to industries able to reuse it, or sequestered underground. The findings in the study represent a possible paradigm shift, Archer said.
“The fact that we’ve designed a carbon capture technology that also generates electricity is, in and of itself, important,” he said. “One of the roadblocks to adopting current carbon dioxide capture technology in electric power plants is that the regeneration of the fluids used for capturing carbon dioxide utilize as much as 25 percent of the energy output of the plant. This seriously limits commercial viability of such technology. Additionally, the captured carbon dioxide must be transported to sites where it can be sequestered or reused, which requires new infrastructure.”
The group reported that their electrochemical cell generated 13 ampere hours per gram of porous carbon (as the cathode) at a discharge potential of around 1.4 volts. The energy produced by the cell is comparable to that produced by the highest energy-density battery systems.
Another key aspect of their findings, Archer says, is in the generation of superoxide intermediates, which are formed when the dioxide is reduced at the cathode. The superoxide reacts with the normally inert carbon dioxide, forming a carbon-carbon oxalate that is widely used in many industries, including pharmaceutical, fiber and metal smelting.
“A process able to convert carbon dioxide into a more reactive molecule such as an oxalate that contains two carbons opens up a cascade of reaction processes that can be used to synthesize a variety of products,” Archer said, noting that the configuration of the electrochemical cell will be dependent on the product one chooses to make from the oxalate.
Al Sadat, who worked on onboard carbon capture vehicles at Saudi Aramco, said this technology in not limited to power-plant applications. “It fits really well with onboard capture in vehicles,” he said, “especially if you think of an internal combustion engine and an auxiliary system that relies on electrical power.”
He said aluminum is the perfect anode for this cell, as it is plentiful, safer than other high-energy density metals and lower in cost than other potential materials (lithium, sodium) while having comparable energy density to lithium. He added that many aluminum plants are already incorporating some sort of power-generation facility into their operations, so this technology could assist in both power generation and reducing carbon emissions.
A current drawback of this technology is that the electrolyte – the liquid connecting the anode to the cathode – is extremely sensitive to water. Ongoing work is addressing the performance of electrochemical systems and the use of electrolytes that are less water-sensitive.
Learn more: Cornell scientists convert carbon dioxide, create electricity
The Latest on: Electrochemical cell
[google_news title=”” keyword=”Electrochemical cell” num_posts=”10″ blurb_length=”0″ show_thumb=”left”]
via Google News
The Latest on: Electrochemical cell
- Green Hydrogen in Transportation: Fueling the Clean Mobility Revolutionon May 1, 2024 at 7:35 am
Green hydrogen is emerging as a clean fuel for transportation, promising to reduce emissions and revolutionize the energy sector.
- Electrochemical sensor selectively detects dangerous bacteriaon April 30, 2024 at 9:00 pm
Researchers at Goethe University Frankfurt and Kiel University have developed a new sensor for the detection of bacteria. It is based on a chip with a surface coating that ensures only very specific ...
- Scientists solve chemical mystery at the interface of biology and technologyon April 30, 2024 at 5:00 pm
At the intersection of biology and technology, researchers are aiming to decipher the cryptic dialects spoken by cells and circuits. With each translation, they bridge the gap between living organisms ...
- Onsite PFAS Destruction: Understanding Electrochemical Oxidationon April 30, 2024 at 12:49 pm
As the need for PFAS treatment continues to emerge, landfills and waste management facilities can rely on electrochemical oxidation as a proven option for ...
- Harnessing Hydrogen: Unveiling Platinum’s Role in Clean Energy Catalystson April 30, 2024 at 10:23 am
Researchers elucidate mechanisms for controlling the surface oxidation processes that affect the performance of platinum catalysts in alkaline media. Platinum (Pt) electrodes are crucial for clean ...
- Researchers achieve electrosynthesis via superwetting organic-solid-water interfaceson April 29, 2024 at 7:11 am
Chinese scientists have recently achieved the direct synthesis of high-purity benzaldehyde chemicals from the selective electrooxidation of benzyl alcohol. The organic-solid-water (OSW) three-phase ...
- Tiny heat pump that relies on changing ambient temperature could be key to powering IoT devices and sensors without batteries forever — Nanoparticles are critical to the ...on April 28, 2024 at 10:46 pm
As IoT technology progresses, the question of how to power these devices, particularly in locations where reliable electrical sources are scarce, presents a significant challenge. Researchers at the ...
- Hydrogen Fuel Cells Market Size Is Projected To Reach USD 16.41 Billion By 2031 | SNS Insideron April 28, 2024 at 2:15 am
( MENAFN - Evertise Digital) - According to SNS Insider, the Hydrogen Fuel Cell M arket valuation to reach USD 16.41 billion by 2031. The estimated CAGR projected for the forecast period of ...
- New sodium battery that can be charged in seconds developedon April 20, 2024 at 11:01 am
Researchers have developed a high-power hybrid sodium-ion battery that can be charged in seconds, potentially replacing lithium-ion batteries.
- New understanding of energy losses in emerging light sourceon April 18, 2024 at 8:34 am
The light-emitting electrochemical cell (LEC) can be fabricated in a sustainable and cost-effective way on both rigid and flexible surfaces making it suitable for a broad range of applications, like ...
via Bing News