UNIST scientists have developed an exiting new catalyst that can split water into hydrogen almost as good as platinum, but less costly and found frequently on Earth.
As described in the journal Nature Nanotechnology, this ruthenium (Ru)-based material works almost as efficient as platinum and likely shows the highest catalytic performance without being affected by the pH of the water.
The research team, led by Professor Jong-Beom Baek of the Energy and Chemical Engineering at UNIST has synthesized Ru and C?N, a two-dimensional organic structure, to verify its performance as a water-splitting catalyst. With the aid of this new catalyst, entitled Ru@C2N it is now possible to efficiently produce hydrogen.
The technology for producing hydrogen from water requires a good catalyst for commercial competitiveness. These water-splitting catalysts must exhibit high hydrogen conversion efficiency and excellent durability, operate well under low-voltage, and should be economical.
The Pt-based catalysts used in the hydrogen generation reaction are highly expensive noble metals, resulting in additional costs and difficulty of mass production. They are also less stable in an alkaline environment.
One solution, many researchers suggest, was to build catalysts made of cheap, non-noble metals. However, because these materials corrode rapidly under acidic condition and operate at very-high voltages, productivity was limited.
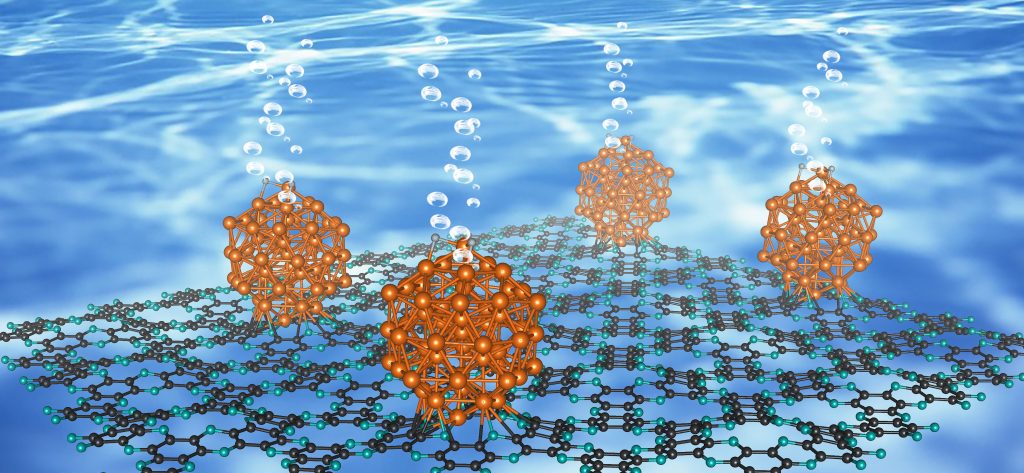
A schematic diagram illustrating the preparation of Ru@C2N is shown in the figure above. (Ruthenium: shown in gold, Carbon: shown in grey , Nitrogen: shown in sky-blue)
The Ru@C?N, developed by Professor Baek is a high-performance material that satisfies all four commercial competitiveness of water-splitting catalysts.
This material exhibits high turnover frequency (TOF) as high as Pt and can be operated on low-voltage supply. In addition, it is not affected by the pH of the water and can be used in any environment.
The synthesis process of Ru@C2N is simple. Professor Baek and his colleagues simply mixed the ruthenium salt (RuCl?) with the monomers which forms the porous two-dimensional organic structure, C?N. The Ru@C2N catalyst is then produced after going through reduction and heat treatment processes.
The researchers used the same process to build M@C2N (M = Co, Ni, Pd, Pt) catalysts, using cobalt (Co), nickel (Ni), lead (Pb) and platinum (Pt). When comparing their efficiency of hydrogen production, the Ru@C2N catalyst exhibited the highest catalytic performance at the lowest overvoltage, as well as superior catalytic activity.
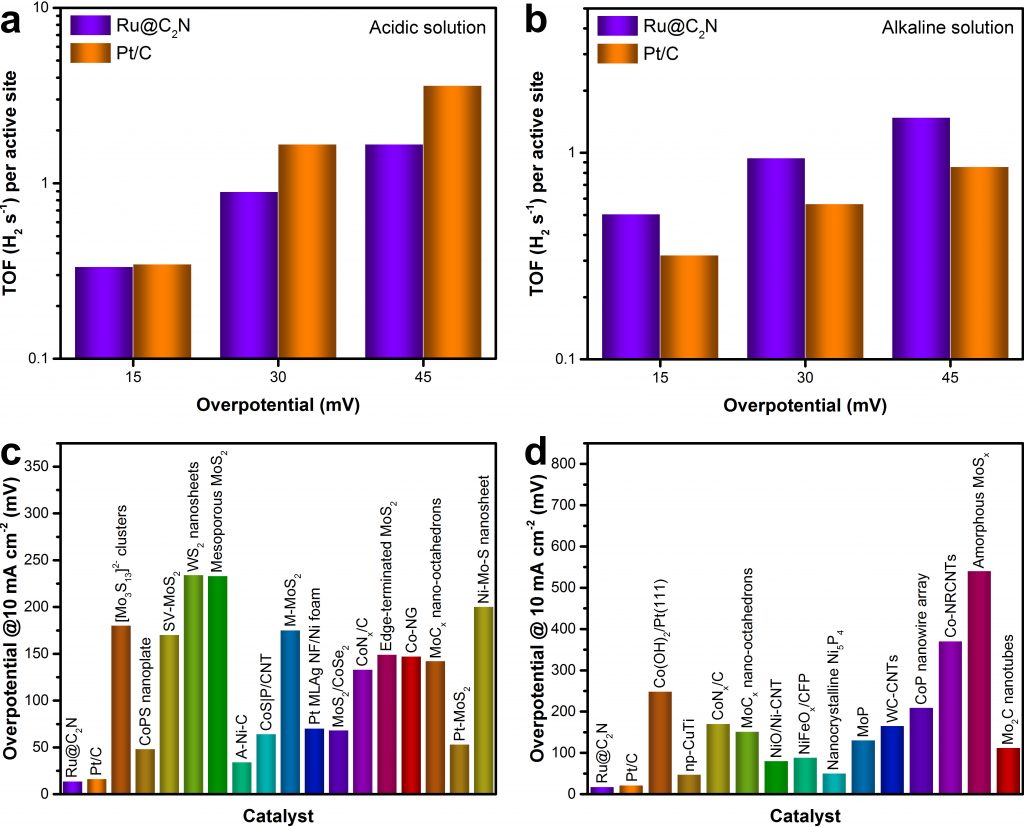
Above figure shows the comparison of the Turnover frequency (TOF) of Ru@C2N with other catalysts.
“Our study not only suggests new directions in materials science, but also presents a wide range of possibilities from basic to applied science,” says Professor Baek. “This material is expected to attract attention in many areas thanks to its scientific potential.”
Learn more: New Economic Water-Splitting Catalyst, Ru@C2N
[osd_subscribe categories=’water-splitting’ placeholder=’Email Address’ button_text=’Subscribe Now for any new posts on the topic “WATER SPLITTING”‘]
Receive an email update when we add a new WATER SPLITTING article.
The Latest on: Water splitting
[google_news title=”” keyword=”water splitting” num_posts=”10″ blurb_length=”0″ show_thumb=”left”]
via Google News
The Latest on: Water splitting
- Ofwat explores Thames Water break-up in ‘Project Telford’ rescue planon April 27, 2024 at 9:00 am
The water regulator is working on rescue plans for Thames Water that could see its sprawling operations dismantled and sold off as piecemeal to rival suppliers.
- NIKKI SIXX On MICK MARS Splitting With MÖTLEY CRÜE: "We Really Had To Sit Down And Go, 'Well, What Do We Do? Do We Fold?'"on April 27, 2024 at 8:26 am
The story of Mick Mars and Mötley Crüe splitting goes back to October 2022, when Mars retired as a touring member of the band. John 5 was announced as his replacement the next day, and was later named ...
- High water hurts early trout seasonon April 26, 2024 at 9:44 pm
Anyone in our area who has ventured out for trout so far this season doesn’t need to be reminded that we have had streams running bank high and dirty a great many days. The opening weekend was a ...
- 84-year-old man found dead in water at Bronx golf course: Policeon April 26, 2024 at 2:57 pm
An 84-year-old man was found dead in a body of water at a golf course in the Bronx, according to police. The body was discovered after a report of a drowning at the Pelham Bay and Split Rock Golf ...
- Lawmakers split on whether amendment including Water Commission needs is relevanton April 25, 2024 at 11:45 am
Disagreement between the two chambers centers on concerns raised by the state attorney general’s office that the amendment to include the Water Commission is not relevant to the original bill.
- Gov. Whitmer Announces $290 Million Boost for Michigan's Water Infrastructure, Supporting Over 4,000 Jobson April 23, 2024 at 12:50 pm
Michigan receives a $290 million budget boost for water infrastructure, promising cleaner water and 4,350 jobs.
- Commentary: Here’s what FWC missed in Split Oak planon April 23, 2024 at 10:55 am
Florida Fish and Wildlife Conservation Commission has a plan for mitigating damage to Split Oak Forest if a toll road is built there, but the plan has no net positive conservation benefit.
- The Sinking Arizona Town Where Water and Politics Collideon April 23, 2024 at 7:20 am
Democrats see an opening to win back rural Trump voters fed up with their groundwater being pumped by huge farms.
- Gov. Gretchen Whitmer announces $290 million to replace lead pipes, upgrade water systems in Michiganon April 22, 2024 at 11:38 am
(CBS DETROIT) - Gov. Gretchen Whitmer and the Department of Environment, Great Lakes, and Energy (EGLE) announced an expansion that will help communities replace lead pipes, upgrade water systems and ...
via Bing News