A team of Berkeley Lab battery researchers led by Elton Cairns has invented an advanced lithium/sulfur (Li/S) cell that, for the first time, offers both long cycle life and a high discharge rate in addition to the inherently low cost and light weight of Li/S batteries.
Description
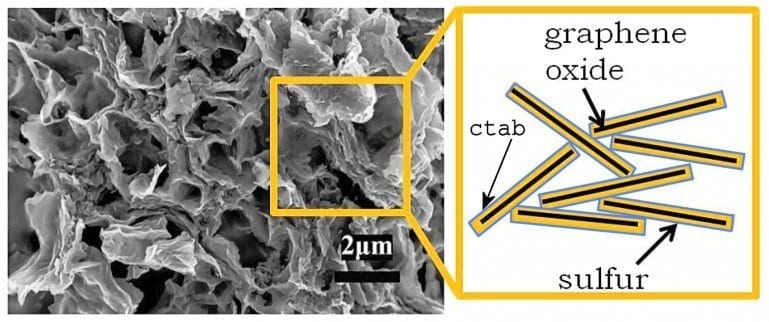
The researchers developed a sulfur-graphene oxide (S-GO) nanocomposite cathode that vastly improves the cycle life of Li/S cells by immobilizing sulfur and inhibiting polysulfides during operation. Tests show that the Berkeley Lab Li/S cell had an estimated specific energy of ~500 Wh/kg, more than double that of current Li-ion cells (~200 Wh/kg). It maintained a high capacity through 1,500 charge/discharge cycles, and also maintained capacity during high discharge and charge rates, such as those required of batteries serving cordless power tools.
In earlier Li/S cells, capacity faded rapidly due to sulfur loss from the cathode by formation of soluble polysulfides in the electrolyte during the charge/discharge cycle. The Berkeley Lab cell avoids this effect through a process that deposits sulfur on ultra-thin cathode surfaces made of nanoporous graphene oxide (GO). Modifying the S-GO composite with a cationic surfactant, CTAB (cetyltrimethyl ammonium bromide) adds a layer of protection for the sulfur attached to the GO.
In addition, a flexible adhesive, or binder, holds the cathode materials in place despite the electromechanical expansion and contraction that occurs during the charge/discharge cycle. Finally, the Berkeley Lab cell employs an improved ionic liquid electrolyte that further reduces polysulfide formation and blocks trace amounts from binding to the lithium metal anodes of the Li/S cells. The combination of features in the Berkeley Lab cell provides an unprecedented level of performance and reliability for Li/S cells.
Benefits
- 1,500 charge/discharge cycles demonstrated
- High specific energy – approximately 500 Wh/kg
- High discharge and charge rates
- Li metal anode: 90 percent less weight than carbon anodes
- Low-cost, Earth-abundant materials
Applications and Industries
- Electric and plug-in hybrid vehicles
- Portable electronics, e.g., mobile phones, cameras
- Cordless power tools
The Latest on: Lithium-sulfur battery
- Batteries Newson April 24, 2024 at 5:00 pm
Researchers are developing new ... Healable Cathode Could Unlock Potential of Solid-State Lithium-Sulfur Batteries Mar. 6, 2024 — Engineers developed a cathode material for lithium-sulfur (Li-S ...
- Storing and utilizing energy with innovative sulfur-based cathodeson April 24, 2024 at 7:31 am
Electric vehicles and portable electronic devices such as laptops and mobile phones are unthinkable without lithium-ion batteries. The problem is highly toxic materials such as cobalt are often used ...
- How Clemson University research could affect your access to lithium ion batterieson April 24, 2024 at 4:06 am
While the availability of lithium iode batteries is expected to wane, researchers are looking to extend that supply by ...
- The rise of low-carbon aircrafton April 23, 2024 at 4:03 pm
The emergence of electric and low-carbon aircraft technologies marks a significant shift in aviation, with substantial growth predicted in the electric aircraft market over the 2030 to 2050 period.
- Researchers Develop Revolutionary Cathode Material for Lithium-Sulfur Batterieson April 19, 2024 at 1:00 pm
Researchers at the University of California - San Diego have developed a self-healing cathode material that overcomes the challenges of traditional sulfur cathodes and could revolutionize solid-state ...
- Fool's Gold May Be Valuable After All After 'Unheard Of' Discoveryon April 19, 2024 at 3:29 am
Scientists found an abundance of lithium in fool's gold—a new source of the much sought-after element which is used in batteries.
- Lithium-Sulfur Battery Market Key Segments, Share, Trends, Size, Growth, and Forecast 2024 to 2032on April 16, 2024 at 6:09 pm
The Lithium-Sulfur (Li-S) battery market is experiencing significant growth and innovation driven by the increasing demand for energy storage solutions across various industries. Li-S batteries are ...
- Sulfur-Based Battery Market Projected to Reach $7.09 billion by 2030 - Exclusive Report by 360iResearchon April 8, 2024 at 5:00 pm
PUNE, India, April 8, 2024 /PRNewswire/ -- The report titled "Sulfur-Based Battery Market by Product Type (Lithium-Sulfur Battery, Sodium-Sulfur Battery), Energy Density (High Energy Density ...
via Bing News
The Latest on: Li/S batteries
- Saudi Arabia set on securing lithium for EV ambitionson April 28, 2024 at 10:31 am
Saudi Arabia is committed to sourcing lithium from overseas as it looks to produce electric vehicle (EV) batteries and invest in the sector, a senior minister said in an interview, noting attempts to ...
- Lithium battery sparks destructive house fire in Franklinon April 26, 2024 at 3:43 pm
A Franklin family is picking up the pieces after a fire ripped through their home Thursday night, killing two animals and causing serious damage to the structure.
- State Fire Marshal: New Tracking Tool Identifies 50 Lithium-Ion Battery Fireson April 26, 2024 at 8:08 am
The Massachusetts Department of Fire Services' new tool for tracking lithium-ion battery fires has helped to identify 50 such incidents in the past six months, more ...
- Renault talks to China's Li Auto and Xiaomi on tech collaborationon April 26, 2024 at 7:14 am
Renault held talks this week with China's Li Auto and Xiaomi on electric and intelligent vehicle technologies, the French carmaker said on Friday, opening the door to potential collaboration on ...
- The Fire That Broke Out at Rivian's Plant in Normal Didn't Involve Battery Componentson April 26, 2024 at 6:29 am
A fire broke out on Thursday on the production line at the Rivian factory in Normal, Illinois, but it didn't have anything to do with Li-ion batteries ...
- A framework to compare lithium battery testing data and results during operationon April 26, 2024 at 3:50 am
Reliably monitoring the amount of lithium (Li) present in rechargeable batteries, specifically in the so-called cathode active material (CAM), is key to understanding the condition of batteries from ...
- CATL's 1,000-km LFP EV battery super-charges at 1 km/secon April 25, 2024 at 8:54 pm
CATL made headlines around the globe last August when it presented the Shenxing battery, an LFP pack capable of adding 400 kilometers (249 miles) of range in a mere 10 minutes at the charger. Not a ...
- On Lithium’s Frontier, Miners Are Betting on a Greener Second Acton April 25, 2024 at 2:00 pm
Despite ailing prices, the battery metal is seen as a climate lifeline for this top-producing Australian region.
- MSC improves lithium battery shipping safety through deal with GSBNon April 25, 2024 at 10:48 am
Maritime freight carrier MSC Mediterranean Shipping Company today took a step to enhance safety in transporting lithium battery shipments by announcing it would collaborate with G ...
- CATL unveils world’s first LFP battery with 4C ultra-fast charging for 370-mi in 10 minson April 25, 2024 at 7:25 am
CATL said the new EV battery is the world’s first with 4C ultra-fast charging and +620 miles (1,000 km) CLTC long-range capabilities. The new battery can gain a one-km range in as little as one minute ...
via Bing News










