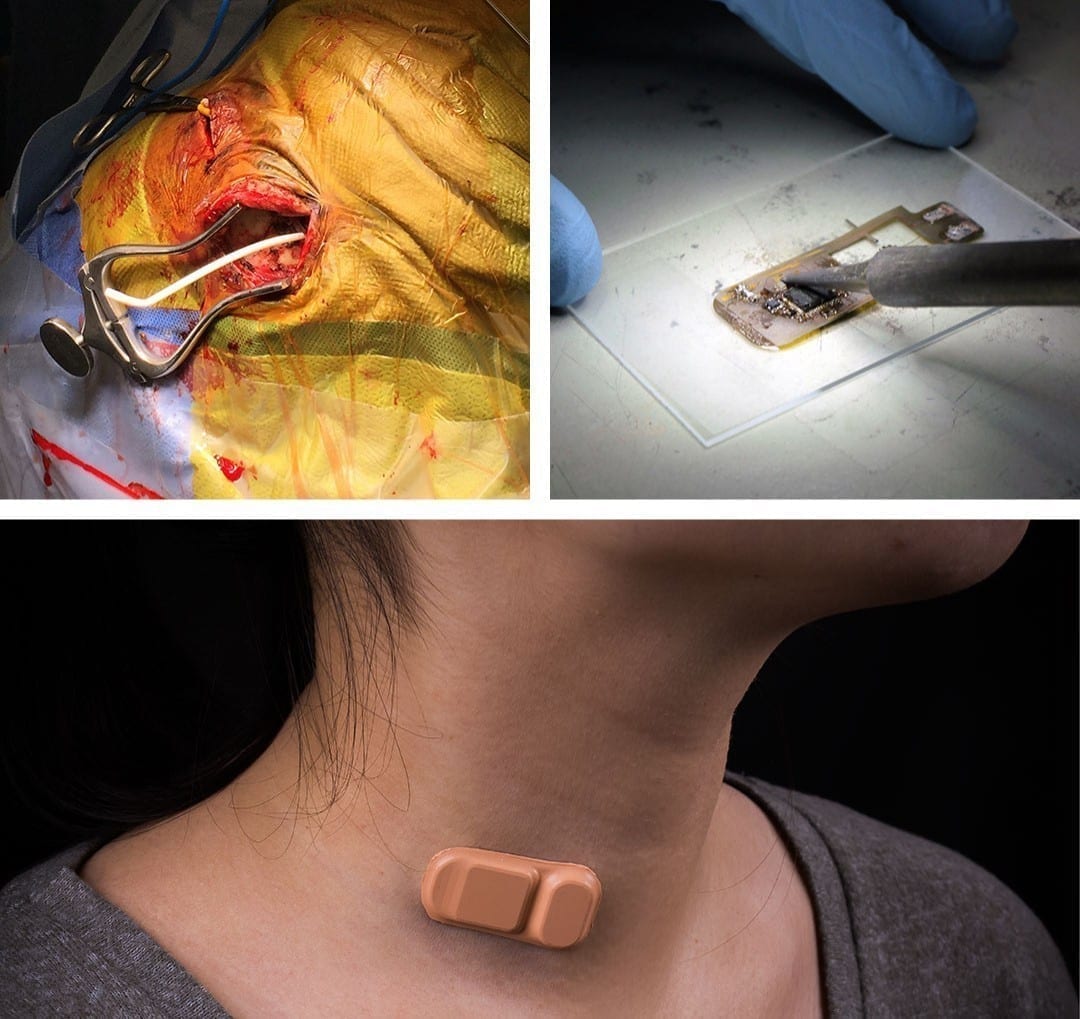
Most people simply take ibuprofen when they get a headache. But for someone with hydrocephalus – a potentially life-threatening condition in which excess fluid builds up in the brain — a headache can indicate a serious problem that can result in a hospital visit, thousands of dollars in scans, radiation and sometimes surgery.
A new wireless, Band-Aid-like sensor developed at Northwestern University could revolutionize the way patients manage hydrocephalus and potentially save the U.S. health care system millions of dollars.
A Northwestern Medicine clinical study successfully tested the device, known as a wearable shunt monitor, on five adult patients with hydrocephalus. The findings were published in Science Translational Medicine.
Hydrocephalus can affect adults and children. Often the child is born with the condition, whereas in adults, it can be acquired from some trauma-related injury, such as bleeding inside the brain or a brain tumor.
The current standard of care involves the surgical implantation of a straw-like catheter known as a ‘shunt,’ which drains the excess fluid out of the brain and into another part of the body.
Shunts have a nearly 100 percent failure rate over 10 years, and diagnosing shunt failure is notoriously difficult. More than a million Americans live with shunts and the constant threat of failure.
The groundbreaking new sensor, developed by the Rogers Research Group at Northwestern, could create immense savings and improve the quality of life for nearly a million people in the U.S. alone.
Impact
- 1 in 1,000 people affected by hydrocephalus
- 100% failure rate of shunts over 10 years
- $50,000 treatment costs per patient per year
When a shunt fails, the patient can experience headaches, nausea and low energy. A patient experiencing any of these symptoms must visit a hospital because if their symptoms are caused by a malfunctioning shunt, it could be life threatening. Once at the hospital, the patient must get a CT scan or an MRI and sometimes must undergo surgery to see if the shunt is working properly.
The new sensor allowed patients in the study to determine within five minutes of placing it on their skin if fluid was flowing through their shunt. The soft and flexible sensor uses measurements of temperature and heat transfer to non-invasively tell if and how much fluid is flowing through.
“We envision you could do this while you’re sitting in the waiting room waiting to see the doctor,” said co-lead author Siddharth Krishnan, a fifth-year Ph.D. student in the Rogers Research Group. “A nurse could come and place it on you and five minutes later, you have a measurement.”
I’m trying to live a normal life, and I really can’t because of the headaches.”
Willie Meyer
Hydrocephalus patient
A device like this would be life changing for Willie Meyer, 26, who has undergone 190 surgeries, spent virtually every holiday in the emergency room and almost missed his high school graduation because of emergency brain surgeries.
Meyer’s mother, Beth, said she learned Willie had hydrocephalus when he was two years old after complaining that “his hair hurt.”
Symptoms of a malfunctioning shunt, such as headaches and fatigue, are similar to symptoms of other illnesses, which causes confusion and stress for caregivers.
“Every time your kid says they have a headache or feels a little sleepy, you automatically think, ‘Is this the shunt?’” said co-senior author Dr. Matthew Potts, assistant professor of neurological surgery at Northwestern University Feinberg School of Medicine and a Northwestern Medicine physician. “We believe that this device can spare patients a lot of the danger and costs of this process.”
Co-lead author Dr. Amit Ayer, who has treated Willie’s hydrocephalus for the last four years, said his patients are a driving force behind his motivation to get the device to market.
“Our patients want to know when they can actually use the device and be part of the trial,” said Ayer, who is a neurosurgery resident at Northwestern Medicine and a student at Kellogg School of Management at Northwestern. “I want to get it out there, so we can help make their lives better.”
How it works
It looks like a Band-Aid that’s talking to a cellphone. There’s nothing like this out there today.”
John A. Rogers
A very small rechargeable battery is built into the sensor. The device is Bluetooth enabled so it can talk to a smartphone and deliver the readings via an Android app. The sensor advances concepts in skin-like “epidermal electronics,” which the Rogers Research Group has been working on for nearly a decade.
It uses a thermal transport measurement, which means the sensor uses tiny amounts of thermal power to minimally increase the temperature of the skin.
If the shunt is working and the excess cerebral spinal fluid is draining properly, the sensor will measure a characteristic heat signature. Similarly, if there is no flow because the shunt has malfunctioned, the sensor will be able to quickly indicate that through heat flow measurements.
The team tested the device in the laboratory before heading to the clinic to perform a pilot study on five patients at Northwestern Memorial Hospital. The team could detect clear differences in cases between measurements over working shunts and on adjacent confusing control locations with no flow.
“This means if someone wants to check if their shunt is working, say, when they have a headache, they can quickly do what we call a ‘spot measurement,’” said co-lead author Tyler Ray, a postdoctoral research fellow in the Rogers Research Group. “This device can also measure flow throughout the day enabling, for the first time, the possibility of continuously monitoring shunt performance. This can lead to important insights into the dynamics of cerebral spinal fluid flow previously inaccessible with current diagnostic tools and flow measurement techniques.”
A larger pediatric clinical trial will be starting soon at Ann & Robert H. Lurie Children’s Hospital of Chicago with the goal of targeting this very vulnerable population. The study authors are also working on outsourced production on the scale of a few hundred sensors to support this study and further develop the technology. Rogers and Ayer are co-founders of Rhaeos, Inc., a company that is commercializing the technology described here.
Learn more: Skin sensor could improve life for a million hydrocephalus patients
The Latest on: Hydrocephalus
[google_news title=”” keyword=”hydrocephalus” num_posts=”10″ blurb_length=”0″ show_thumb=”left”]
via Google News
The Latest on: Hydrocephalus
- Eighteen set to be inducted into Lockport's Howell Motors Hockey Hall of Fameon May 1, 2024 at 3:40 pm
The Howell Motors Hockey Hall of Fame is set to induct a new batch of key figures for the ninth time. The ceremony will be held Saturday at the Cornerstone Arena. Doors open at 7 p.m. and the ceremony ...
- This Morning's Michelle Elman gets 'signature' manicure after splitting from cheating fiancé the day he proposed as she quips 'when the nails last longer than the engagement'on May 1, 2024 at 7:55 am
She had a brain tumour, a punctured intestine, an obstructed bowel, a cyst in her brain and a condition called hydrocephalus - where fluid builds up in the brain, causing, dangerous pressure that can ...
- The Diagnosis and Treatment of Idiopathic Normal Pressure Hydrocephaluson April 30, 2024 at 5:00 pm
Idiopathic normal pressure hydrocephalus (INPH) is a syndrome that is characterized by gait impairment, cognitive decline and urinary incontinence, and is associated with ventriculomegaly in the ...
- Cork man who has lived life in the face of adversity launches first bookon April 30, 2024 at 3:45 am
You shouldn’t waste the short time you have on this beautiful planet,” reads the last line of a new book written by a Cork man who has lived his life against the odds.
- Second puppy rescued from streets of Dublin sadly dies just days after sisteron April 29, 2024 at 7:10 am
A second puppy rescued from the streets of Dublin has sadly died just days after his sister.
- Devastated This Morning star splits from fiancé after he cheated on her as she reveals the horrible way she found outon April 29, 2024 at 3:16 am
THIS Morning star Michelle Elman has revealed she’s split from her fiancé after he cheated on her. The body confidence coach and author shared her ordeal with her followers through tears ...
- The Diagnosis and Treatment of Idiopathic Normal Pressure Hydrocephaluson April 28, 2024 at 5:00 pm
Disclosure: In the 12 months prior to the submission of this manuscript (March 2006), MA Williams and D Rigamonti received financial support for hydrocephalus research from Medtronic, and Dr ...
- Professional Faqs: What Are The Complications Hydrocephalus?on April 18, 2024 at 12:09 am
Expert opinion from Mohan P. Abraham M.D., FAAFP (Family Physician) · 40 years of experience · USA The possible complications of hydrocephalus include : 1) Visual changes, occlusion of posterior ...
- Ask A Doctor: What Are The Complications Hydrocephalus?on April 17, 2024 at 11:26 pm
It will depend on the cause and the patient’s age that hydrocephalus appears. In newborns, it can cause significant neurological damage and developmental delays. In adults, it may cause visual changes ...
via Bing News