The discovery that water microdroplets can replace potentially toxic agents in the creation of gold nanoparticles and nanowires could help usher in a new era of “green chemistry.”
An experiment that, by design, was not supposed to turn up anything of note instead produced a “bewildering” surprise, according to the Stanford scientists who made the discovery: a new way of creating gold nanoparticles and nanowires using water droplets.
The technique, detailed April 19 in the journal Nature Communications, is the latest discovery in the new field of on-droplet chemistry and could lead to more environmentally friendly ways to produce nanoparticles of gold and other metals, said study leader Richard Zare, a chemist in the School of Humanities and Sciences and a co-founder of Stanford Bio-X.
“Being able to do reactions in water means you don’t have to worry about contamination. It’s green chemistry,” said Zare, who is the Marguerite Blake Wilbur Professor in Natural Science at Stanford.
Noble metal
Gold is known as a noble metal because it is relatively unreactive. Unlike base metals such as nickel and copper, gold is resistant to corrosion and oxidation, which is one reason it is such a popular metal for jewelry.
Around the mid-1980s, however, scientists discovered that gold’s chemical aloofness only manifests at large, or macroscopic, scales. At the nanometer scale, gold particles are very chemically reactive and make excellent catalysts. Today, gold nanostructures have found a role in a wide variety of applications, including bio-imaging, drug delivery, toxic gas detection and biosensors.
Until now, however, the only reliable way to make gold nanoparticles was to combine the gold precursor chloroauric acid with a reducing agent such as sodium borohydride.
The reaction transfers electrons from the reducing agent to the chloroauric acid, liberating gold atoms in the process. Depending on how the gold atoms then clump together, they can form nano-size beads, wires, rods, prisms and more.
A spritz of gold
Recently, Zare and his colleagues wondered whether this gold-producing reaction would proceed any differently with tiny, micron-size droplets of chloroauric acid and sodium borohydide. How large is a microdroplet? “It is like squeezing a perfume bottle and out spritzes a mist of microdroplets,” Zare said.
From previous experiments, the scientists knew that some chemical reactions proceed much faster in microdroplets than in larger solution volumes.
Indeed, the team observed that gold nanoparticle grew over 100,000 times faster in microdroplets. However, the most striking observation came while running a control experiment in which they replaced the reducing agent – which ordinarily releases the gold particles – with microdroplets of water.
“Much to our bewilderment, we found that gold nanostructures could be made without any added reducing agents,” said study first author Jae Kyoo Lee, a research associate.
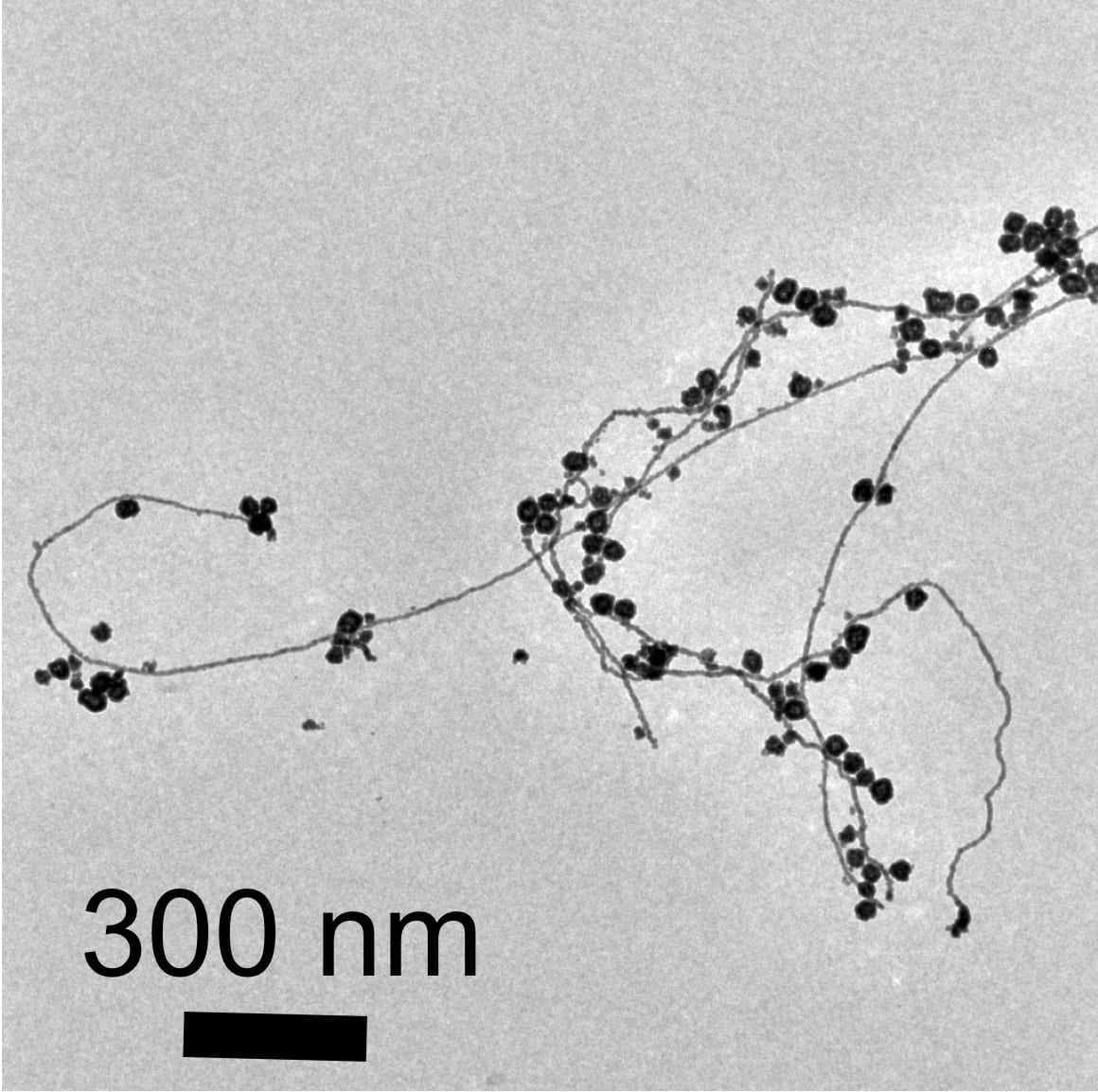
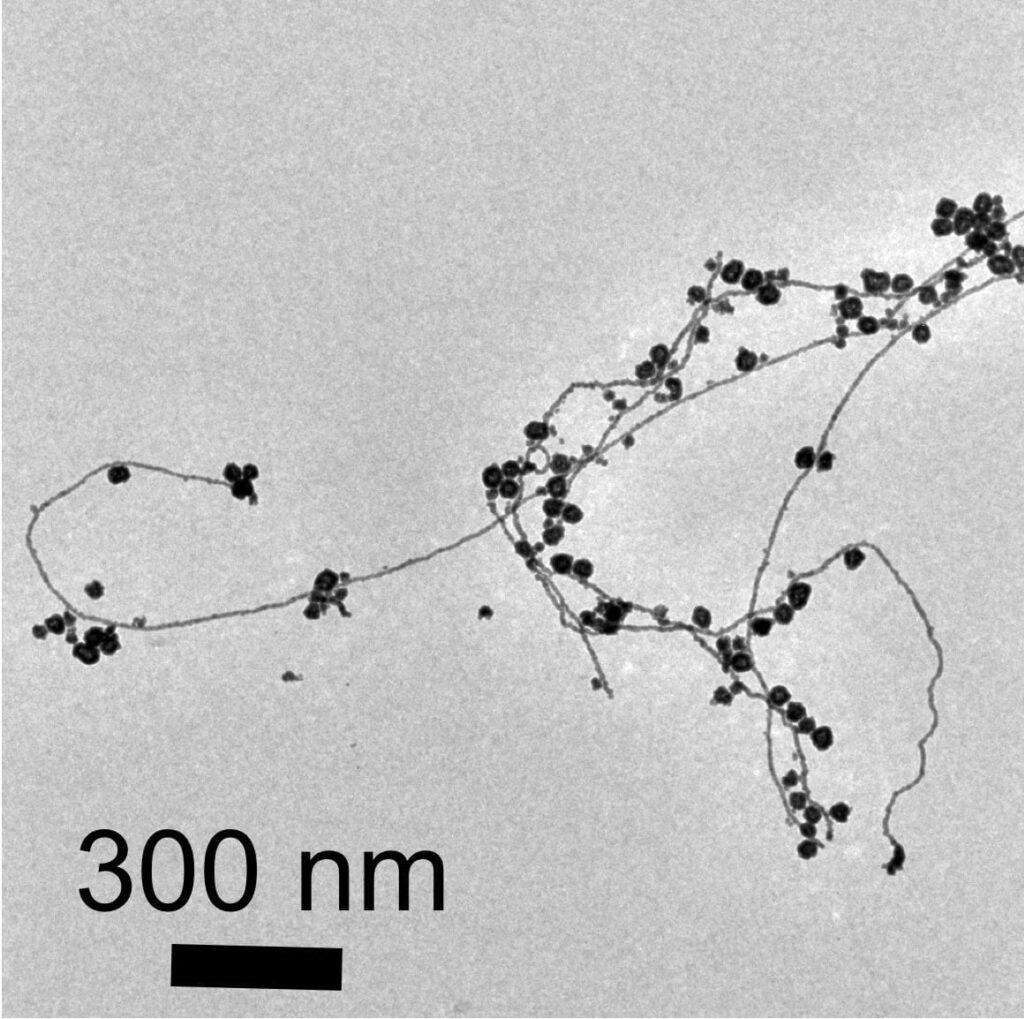
Viewed under an electron microscope, the gold nanoparticles and nanowires appear fused together like berry clusters on a branch.
The surprise finding means that pure water microdroplets can serve as microreactors for the production of gold nanostructures. “This is yet more evidence that reactions in water droplets can be fundamentally different from those in bulk water,” said study coauthor Devleena Samanta, a former graduate student in Zare’s lab and co-author on the paper.
If the process can be scaled up, it could eliminate the need for potentially toxic reducing agents that have harmful health side effects or that can pollute waterways, Zare said.
It’s still unclear why water microdroplets are able to replace a reducing agent in this reaction. One possibility is that transforming the water into microdroplets greatly increases its surface area, creating the opportunity for a strong electric field to form at the air-water interface, which may promote the formation of gold nanoparticles and nanowires.
“The surface area atop a one-liter beaker of water is less than one square meter. But if you turn the water in that beaker into microdroplets, you will get about 3,000 square meters of surface area – about the size of half a football field,” Zare said.
The team is exploring ways to utilize the nanostructures for various catalytic and biomedical applications and to refine their technique to create gold films.
“We observed a network of nanowires that may allow the formation of a thin layer of nanowires,” Samanta said.
Learn more: Stanford scientists create gold nanoparticles in water
The Latest on: Green chemistry
[google_news title=”” keyword=”green chemistry” num_posts=”10″ blurb_length=”0″ show_thumb=”left”]
via Google News
The Latest on: Green chemistry
- Proposals sought for grants for green chemistry pharma researchon April 25, 2024 at 5:00 pm
The ACS Green Chemistry Institute Pharmaceutical Roundtable is seeking to fund research to advance green chemistry solutions to key challenges in pharmaceutical process development and production.
- Proposals sought for grants for green chemistry pharma researchon April 25, 2024 at 5:00 pm
The ACS Green Chemistry Institute Pharmaceutical Roundtable is seeking to fund research to advance green chemistry solutions to key challenges in pharmaceutical process development and production.
- Beyond Benign and Dow Expand Collaboration To Advance Green Chemistry Educationon April 24, 2024 at 7:55 am
Dow (NYSE: DOW), a global leader in materials science, and Beyond Benign, a nonprofit organization focused on making green chemistry an integral part of education, announce an expanded multi-year ...
- Beyond Benign, MilliporeSigma partner on green chemistryon April 3, 2024 at 5:00 pm
In October 2023, due to the support of MilliporeSigma and the American Chemical Society, Beyond Benign launched its online platform — the Green Chemistry Teaching and Learning Community.
- What Is Green Chemistry?on January 31, 2024 at 4:10 am
Green chemistry takes the EPA's mandate a step further and creates a new reality for chemistry and engineering by asking chemists and engineers to design chemicals, chemical processes and commercial ...
- Why every industry needs green chemistryon December 11, 2023 at 1:55 pm
Only part of the resources that are extracted from the Earth are transformed into products; most end up as waste, says Buxing Han, a green chemist at the Institute of Chemistry, Chinese Academy of ...
- Green Chemistry Student Awardson March 22, 2023 at 1:01 pm
The ACS Green Chemistry Institute (GCI) awards recognize students for excellence in research and provide monetary support for travel so they can gain valuable experience presenting their green ...
- M.S. or Ph.D. in Polymer Chemistryon February 10, 2023 at 10:15 am
[email protected] polymer chemistry, advanced supramolecular catalysis for "green" chemistry, polymer synthesis through enzymatic biocatalysis, hydrogels and networks for additive manufacturing, drug ...
- Green Chemistryon December 22, 2022 at 12:23 am
Green Chemistry provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. Green Chemistry is at the frontiers of this ...
via Bing News