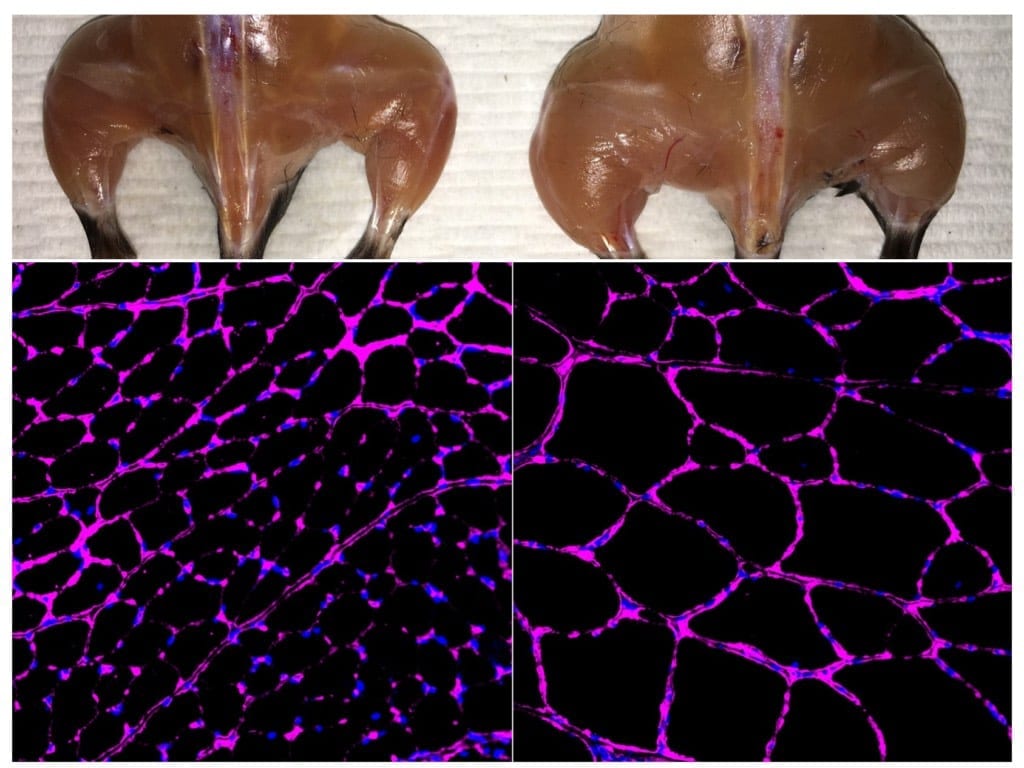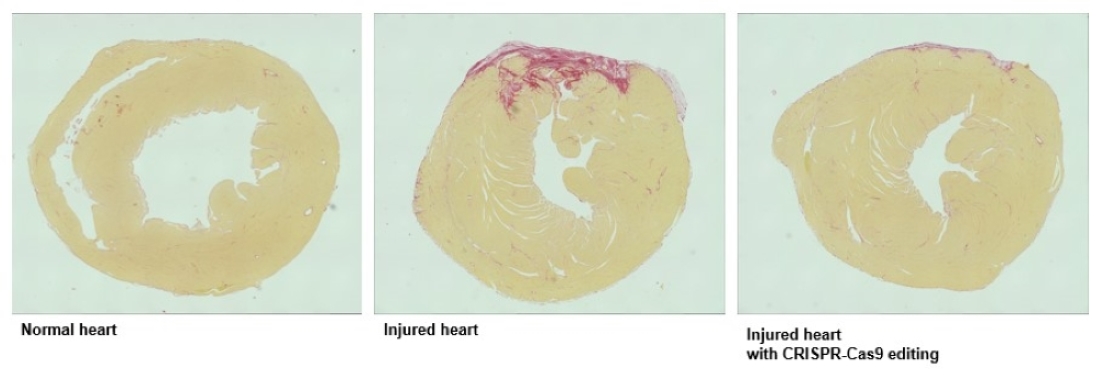
The figure shows cross-sections of mouse hearts with areas of damage in red. Treatment with virus-expressing CRISPR components reduces cardiac damage following ischemic injury.
Editing a gene that prompts a cascade of damage after a heart attack appeared to reverse this inevitable course in mice, leaving their hearts remarkably unharmed, a new study by UT Southwestern scientists showed. The findings, published in Science, could lead to a new strategy for protecting patients from the consequences of heart disease.
“Usually, depriving the heart of oxygen for an extended period, as often happens in a heart attack, will damage it substantially. But those animals whose heart muscles were subjected to gene editing after induced heart attacks seem to be essentially normal in the weeks and months afterward,” said Eric Olson, Ph.D., Director of the Hamon Center for Regenerative Science and Medicine and Chair of Molecular Biology at UTSW, who co-led the study with Rhonda Bassel-Duby, Ph.D., Professor of Molecular Biology.
Since its discovery a decade ago, the CRISPR-Cas9 gene editing system has been used by scientists to correct genetic mutations responsible for disease, including work by the Olson lab on Duchenne muscular dystrophy. However, Dr. Bassel-Duby explained, these diseases caused by mutations affect relatively small groups of people, whereas nongenetic diseases affect far larger numbers. For example, cardiovascular diseases are the leading cause of death globally, killing about 19 million people every year.
Researchers recently discovered that much of the damage from a heart attack – an event characterized by blockage of blood vessels that feed the heart, depriving it of oxygen – is caused by overactivation of a gene called CaMKII?. This gene plays a variety of roles in heart cell signaling and function. The overactivation occurs when the heart is stressed, prompted by oxidation of two methionine amino acids that form part of the CaMKII? protein.
Drs. Olson and Bassel-Duby and their colleagues reasoned that if these methionines could be converted to a different amino acid instead, oxidation wouldn’t occur, sparing the heart from CaMKII? overactivation and subsequent damage after a heart attack.
To test this idea, Simon Lebek, M.D., a postdoctoral fellow, and other members of the team used CRISPR-Cas9 to edit CaMKII? in human heart cells growing in a petri dish. Tests showed that when unedited heart cells were placed into a low-oxygen chamber, they developed numerous markers of damage and subsequently died. However, the edited cells were protected from damage and survived.
The researchers then tried a similar experiment in live mice, inducing a heart attack in these animals by restricting blood flow to their heart’s main pumping chamber for 45 minutes and then delivering CaMKII? gene editing components directly to some animals’ hearts. Both mice that received gene editing and those that did not had severely compromised heart function in the first 24 hours after their heart attacks. But while the mice without the gene editing continued to worsen over time, those that received gene editing steadily improved over the next few weeks, ultimately achieving cardiac function that was nearly indistinguishable from before their heart attacks.
Further research showed that the gene editing appeared to be isolated to the heart – there was no evidence of edited CaMKII? in other organs, including the liver, brain, or muscles. No negative side effects were apparent almost a year out from treatment, Drs. Olson and Bassel-Duby said. The treatment also appeared to be durable, they added, noting that the gene-edited mice were able to do heavy exercise similar to mice that never had heart attacks.
Although this treatment will need substantial safety and efficacy studies before it can be used in humans, the researchers suggest that gene editing could offer a promising solution for treating patients in the aftermath of a heart attack and could have potential for a range of other nongenetic diseases.
Original Article: Gene editing halts damage in mice after heart attacks in UT Southwestern study
More from: University of Texas Southwestern Medical Center
The Latest Updates from Bing News
Go deeper with Bing News on:
CRISPR-Cas9
- CRISPR is promising to tackle antimicrobial resistance, but bacteria can fight back
In his presentation "How to use CRISPR-Cas to combat AMR" at the ESCMID Global Congress, Assistant Prof. Ibrahim Bitar, Department of Microbiology, Faculty of Medicine and University Hospital in Plzen ...
- New Zealand: How gene technology is changing future of food
Opinion: Ethical use of genetic tech may accelerate the adoption of new crops and animals.
- Buy Rating on Crispr Therapeutics AG Backed by Exa-cel’s Precision and Favorable Safety Profile
Salim Syed, an analyst from Mizuho Securities, maintained the Buy rating on Crispr Therapeutics AG (CRSP – Research Report). The ...
- This AI Just Designed a More Precise CRISPR Gene Editor for Human Cells From Scratch
Based on large language models—the tech behind the popular ChatGPT—Profluent's AI designed a new gene editor and put it to work in human cells.
- Peninsula biotech co-founded by Jennifer Doudna lands CRISPR gene-editing pact with Regeneron
The big deal between Regeneron and a Peninsula biotech co-founded by Jennifer Doudna is also a small deal. And that’s a huge deal.
Go deeper with Bing News on:
Gene editing
- Walgreens expands specialty pharmacy with unit dedicated to cell and gene therapies
Walgreens Boots Alliance (NASDAQ:WBA) has expanded its specialty pharmacy services with a unit dedicated to gene and cell therapy services, the company announced late Wednesday. Effective Aug. 1, the new Walgreens Specialty Pharmacy business will replace the healthcare giant's existing specialty pharmacy offering,
- Generative A.I. Arrives in the Gene Editing World of CRISPR
Much as ChatGPT generates poetry, a new A.I. system devises blueprints for microscopic mechanisms that can edit your DNA.