via University of Houston
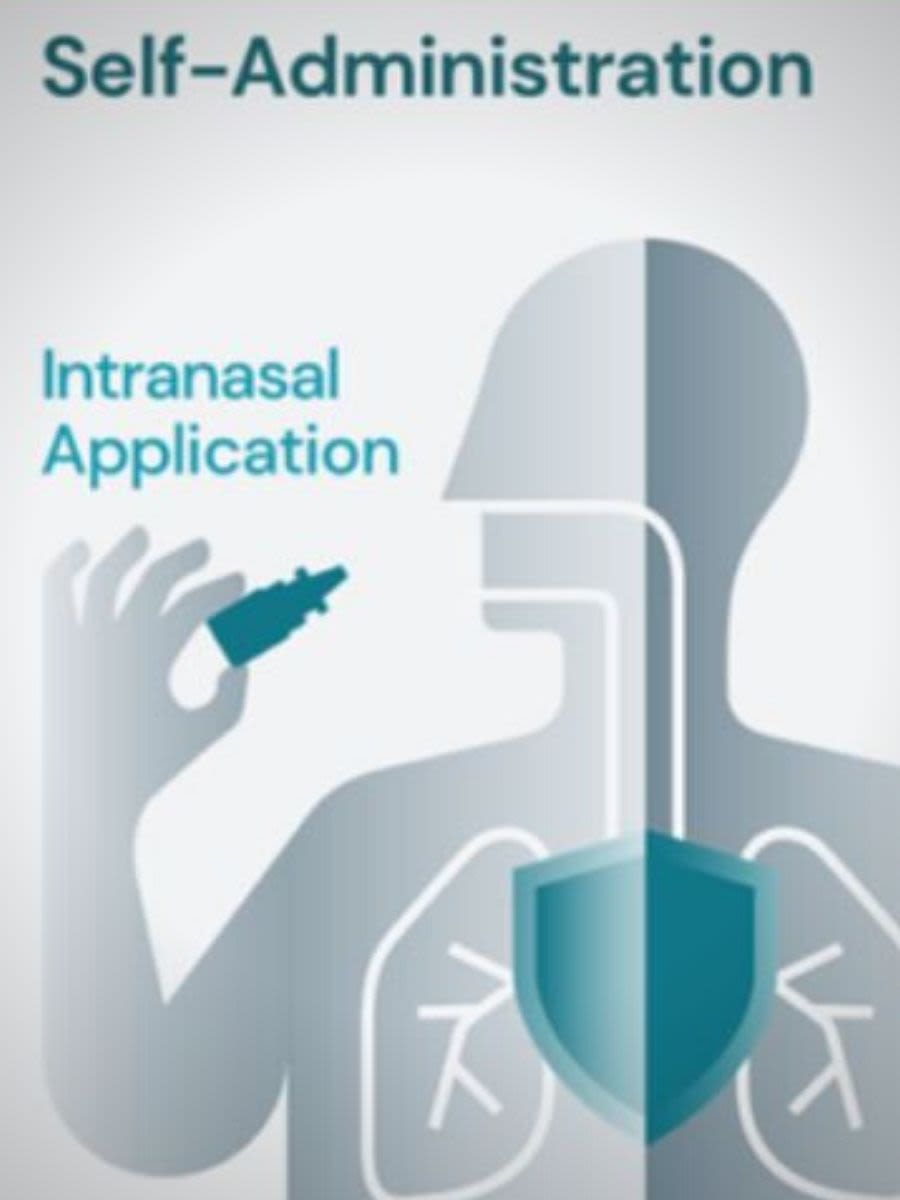
Breathe in, breathe out. That’s how easy it is for SARS-CoV-2, the virus that causes COVID-19, to enter your nose. And though remarkable progress has been made in developing intramuscular vaccines against SARS-CoV- 2, such as the readily available Pfizer, Moderna and Johnson & Johnson vaccines, nothing yet – like a nasal vaccine – has been approved to provide mucosal immunity in the nose, the first barrier against the virus before it travels down to the lungs.
But now, we’re one step closer.
Navin Varadarajan, University of Houston M.D. Anderson Professor of Chemical and Biomolecular Engineering, and his colleagues, are reporting in iScience the development of an intranasal subunit vaccine that provides durable local immunity against inhaled pathogens.
“Mucosal vaccination can stimulate both systemic and mucosal immunity and has the advantagggggggggge of being a non-invasive procedure suitable for immunization of large populations,” said Varadarajan. “However, mucosal vaccination has been hampered by the lack of efficient delivery of the antigen and the need for appropriate adjuvants that can stimulate a robust immune response without toxicity.”
“Our results show that the candidate vaccine formulation is safe, produces rapid immune responses – within seven days – and elicits comprehensive immunity against SARS-CoV-2.”Navin Varadarajan, lead study author
To solve those problems, Varadarajan collaborated with Xinli Liu, associate professor of pharmaceutics at the UH College of Pharmacy, and an expert in nanoparticle delivery. Liu’s team was able to encapsulate the agonist of the stimulator of interferon genes (STING) within liposomal particles to yield the adjuvant named NanoSTING. The function of the adjuvant is to promote the body’s immune response.
“NanoSTING has a small particle size around 100 nanometers which exhibits significantly different physical and chemical properties to the conventional adjuvant,” said Liu.
“We used NanoSTING as the adjuvant for intranasal vaccination and single-cell RNA-sequencing to confirm the nasal-associated lymphoid tissue as an inductive site upon vaccination. Our results show that the candidate vaccine formulation is safe, produces rapid immune responses – within seven days – and elicits comprehensive immunity against SARS-CoV-2,” said Varadarajan.
“Equitable distribution requires vaccines that are stable and that can be shipped easily.”
A fundamental limitation of intramuscular vaccines is that they are not designed to elicit mucosal immunity. As prior work with other respiratory pathogens like influenza has shown, sterilizing immunity to virus re-infection requires adaptive immune responses in the respiratory tract and the lung.
The nasal vaccine will also serve to equitably distribute vaccines worldwide, according to the researchers. It is estimated that first world countries have already secured and vaccinated multiple intramuscular doses for each citizen while billions of people in countries like India, South Africa, and Brazil with large outbreaks are currently not immunized. These outbreaks and viral spread are known to facilitate viral evolution leading to decreased efficacy of all vaccines.
“Equitable distribution requires vaccines that are stable and that can be shipped easily. As we have shown, each of our components, the protein (lyophilized) and the adjuvant (NanoSTING) are stable for over 11 months and can be stored and shipped without the need for freezing,” said Varadarajan.
Varadarajan is co-founder of AuraVax Therapeutics Inc., a pioneering biotech company developing novel intranasal vaccines and therapies to help patients defeat debilitating diseases, including COVID-19. The company has an exclusive license agreement with UH with respect to the intellectual property covering intranasal vaccines and STING agonist technologies. They have initiated the manufacturing process and plan to engage the FDA later this year.
Original Article: IN THROUGH THE NOSE…
More from: University of Houston | University of Texas MD Anderson Cancer Center
The Latest Updates from Bing News & Google News
Go deeper with Bing News on:
Nasal vaccine
- Scientists tried to give people COVID — and failedon April 30, 2024 at 5:00 pm
Researchers deliberately infect participants with SARS-CoV-2 in ‘challenge’ trials — but high levels of immunity complicate efforts to test vaccines and treatments.
- Predictive Oncology Announces Significant Progress with FluGen In the Development of First-of-Its-Kind Intranasal Flu Vaccineon April 30, 2024 at 1:59 pm
PITTSBURGH, April 30, 2024 (GLOBE NEWSWIRE) -- Predictive Oncology Inc. (NASDAQ: POAI), a leader in AI-driven drug discovery and biologics, announces a collaboration with FluGen to bring a ...
- Predictive Oncology stock rallies 30% post-market on vaccine collaborationon April 30, 2024 at 1:45 pm
Shares of Predictive Oncology (NASDAQ:POAI) shot up nearly 30% in post-market trading Tuesday after the company said it was collaborating with FluGen to bring its intranasal flu vaccine to market.
- Hong Kong residents urged to get influenza vaccines after the death of a third child this yearon April 29, 2024 at 5:00 pm
Hong Kong health officials are reaching out to city residents, telling them to get themselves vaccinated against the flu after a third child died from the infection this year. Authorities have warned ...
- Nasal vaccines could help stop Covid: WHOon April 28, 2024 at 5:00 pm
And India approved a nasally-administered Covid-19 vaccine for emergency use on Tuesday, developed by Bharat Biotech. WHO emergencies director Mike Ryan said nasal vaccines generated immune ...
Go deeper with Google Headlines on:
Nasal vaccine
[google_news title=”” keyword=”nasal vaccine” num_posts=”5″ blurb_length=”0″ show_thumb=”left”]
Go deeper with Bing News on:
Mucosal vaccination
- Everest Medicines Announces Hong Kong Department of Health Approval of Nefecon® for the Treatment for Primary IgA Nephropathy in Adult Patientson May 1, 2024 at 6:23 pm
Everest Medicines (HKEX 1952.HK, "Everest", or the "Company") announced that the Hong Kong Department of Health had approved Nefecon ® for the treatment of primary immunoglobulin A nephropathy (IgAN) ...
- Why Africa is facing an uphill battle to make its own vaccineson May 1, 2024 at 6:30 am
The continent – in dire need of its own production capabilities – could once again be at the back of the vaccine queue in the next pandemic ...
- Vaccine Developers Leverage mRNA and Other Powerful Technologieson April 30, 2024 at 5:00 pm
attenuated virus design with codon deoptimization to develop synthetic biology–based vaccine solutions. Using live, attenuated viruses can be advantageous against respiratory infections, facilitating ...
- Predictive Oncology Announces Significant Progress with FluGen In the Development of First-of-Its-Kind Intranasal Flu Vaccineon April 30, 2024 at 1:59 pm
PITTSBURGH, April 30, 2024 (GLOBE NEWSWIRE) -- Predictive Oncology Inc. (NASDAQ: POAI), a leader in AI-driven drug discovery and biologics, announces a collaboration with FluGen to bring a ...
- Study rethinks immune system strategies to boost vaccine effectivenesson April 28, 2024 at 9:52 pm
A recent Cell journal review explores key immunological assumptions to enhance our understanding of vaccine design, autoimmune responses, and immune system pathology, aiming to refine intervention ...
Go deeper with Google Headlines on:
Mucosal vaccination
[google_news title=”” keyword=”mucosal vaccination” num_posts=”5″ blurb_length=”0″ show_thumb=”left”]