All good research breaks new ground, but rarely does the research unearth truths that challenge the foundation of a science.
That’s what Artem R. Oganov has done, and the professor of theoretical crystallography in the Department of Geosciences will have his work published in the Dec. 20 issue of the journal Science.
The paper titled “Unexpected stable stoichiometries of sodium chlorides,” documents his predictions about, and experiments in, compressing sodium chloride—rock salt—to form new compounds. These compounds validate his methodology for predicting the properties of objects—a methodology now used worldwide for computational material discovery—and hold the promise of novel materials and applications.
“I think this work is the beginning of a revolution in chemistry,” Oganov says. “We found, at low pressures achievable in the lab, perfectly stable compounds that contradict the classical rules of chemistry. If you apply the rather modest pressure of 200,000 atmospheres—for comparison purposes, the pressure at the center of the earth is 3.6 million atmospheres—everything we know from chemistry textbooks falls apart.”
Standard chemistry textbooks say that sodium and chlorine have very different electronegativities, and thus must form an ionic compound with a well-defined composition. Sodium’s charge is +1, chlorine’s charge is -1; sodium will give away an electron, chlorine wants to take an electron. According to chemistry texts and common sense, the only possible combination of these atoms in a compound is 1:1—rock salt, or NaCl.
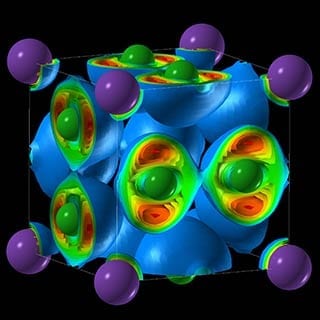
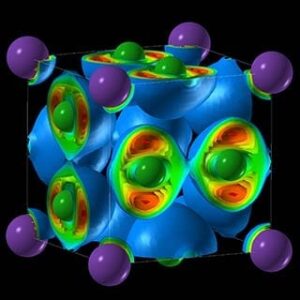
“We found crazy compounds that violate textbook rules—NaCl3, NaCl7, Na3Cl2, Na2Cl, and Na3Cl,” says Weiwei Zhang, the lead author and visiting scholar at the Oganov lab and Stony Brook’s Center for Materials by Design, directed by Oganov. “These compounds are thermodynamically stable and, once made, remain indefinitely; nothing will make them fall apart. Classical chemistry forbids their very existence. Classical chemistry also says atoms try to fulfill the octet rule—elements gain or lose electrons to attain an electron configuration of the nearest noble gas, with complete outer electron shells that make them very stable. Well, here that rule is not satisfied.”
This opens all kinds of possibilities. Oganov posited that, if you mix NaCl with metallic sodium, compress in a diamond anvil cell, and heat, you will get sodium-rich compounds like Na3Cl. He likewise theorized that, if you take NaCl, mix it with pure chlorine, and compress and heat, you will get chlorine-rich compounds such as NaCl3. This is exactly what was seen in the experiments, which were performed by the team of Alexander F. Goncharov of Carnegie Institution of Washington, confirming Oganov’s predictions. “When you change the theoretical underpinnings of chemistry, that’s a big deal,” Goncharov says. “But what it also means is that we can make new materials with exotic properties.”
Among the compounds Oganov and his team created are two-dimensional metals, where electricity is conducted along the layers of the structure. “One of these materials—Na3Cl—has a fascinating structure,” he says. “It is comprised of layers of NaCl and layers of pure sodium. The NaCl layers act as insulators; the pure sodium layers conduct electricity. Systems with two-dimensional electrical conductivity have attracted a lot of interest.”
Like much of science, Oganov’s pursuit began with curiosity—and obstinacy.
The Latest on: Novel materials
[google_news title=”” keyword=”Novel materials” num_posts=”10″ blurb_length=”0″ show_thumb=”left”]
via Google News
The Latest on: Novel materials
- Materials scientists reveal pathway for designing optical materials with specialized propertieson May 7, 2024 at 11:51 am
a graduate student working with Mishra in WashU's Institute of Materials Science and Engineering, describe a new pathway to obtain novel optical and electronic properties from structural disorder.
- Indium Corporation to Showcase HIA Materials at ECTCon May 7, 2024 at 8:56 am
Indium Corporation’s proven SiPaste® series is specifically designed for fine feature printing with fine powders ranging from Type 5 to Type 8. They help Avoid the Void®, reduce slumping, and ...
- Teknova Launches Proprietary Build-Tek™ Solutions Custom Configurator and Latest AAV-Tek™ Product to Further Accelerate Novel Therapy Developmenton May 7, 2024 at 3:00 am
All buffers are made in an ISO 13485 certified facility using high-quality ingredients, and all raw materials and finished products are ... industry to accelerate the discovery and development of ...
- New high-throughput device to unlock the potential of advanced materialson May 6, 2024 at 12:33 pm
A Birmingham researcher has developed a new high-throughput device that produces libraries of nanomaterials using sustainable mechanochemical approaches.
- UW Researchers Develop Novel Technology for Distinguishing Elements at Subnanometer Scaleon May 5, 2024 at 5:00 pm
A University of Wyoming research group has demonstrated a novel method to distinguish elements at the ... High entropy alloys and materials exhibit great potential for many applications, including ...
- Synthesis of New Materials Challenge worth $Millions from TRIon May 5, 2024 at 12:23 pm
Toyota Research Institute (TRI) announces a multiyear, multimillion-dollar challenge aimed at closing the gap between recent advances in AI prediction of new materials and finding the actual “recipe” ...
- Revolutionizing Photonics: 2D Materials Manipulate Light With Remarkable Precisionon May 4, 2024 at 3:37 pm
NYU Abu Dhabi researchers have unveiled a novel 2D material improving optical modulation for advanced systems and communications. Responding to the increasing demand for efficient, tunable optical mat ...
- Novel fabrication method creates aligned nanofiber hydrogels for tissue regenerationon May 3, 2024 at 10:12 pm
A team of chemists and bioengineers at Rice University and the University of Houston have achieved a significant milestone in their work to create a biomaterial that can be used to grow biological ...
- Ecogensus Develops Novel New Sustainable Resource: Plastic Substitutes Derived from Mixed Household Wasteson May 2, 2024 at 6:15 pm
New Patent Grant Elevates Ecogensus as a Leader in Advanced Material Science – Transforming Everyday Waste into High-Performance Polymer Composite Powders HOUSTON, May 3, 2024 /PRNewswire/ -- ...
- The Novel Material Revolutionizing Energy Storageon April 28, 2024 at 10:00 am
Washington University in St. Louis scientists have developed a novel material that supercharges innovation in electrostatic energy storage, with the potential to revolutionize electronic devices.
via Bing News