The new anodes are made from low-cost materials,
Lithium-ion batteries are everywhere, in smart phones, laptops, an array of other consumer electronics, and the newest electric cars. Good as they are, they could be much better, especially when it comes to lowering the cost and extending the range of electric cars. To do that, batteries need to store a lot more energy.
The anode is a critical component for storing energy in lithium-ion batteries. A team of scientists at the U.S. Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab) has designed a new kind of anode that can absorb eight times the lithium of current designs, and has maintained its greatly increased energy capacity after over a year of testing and many hundreds of charge-discharge cycles.
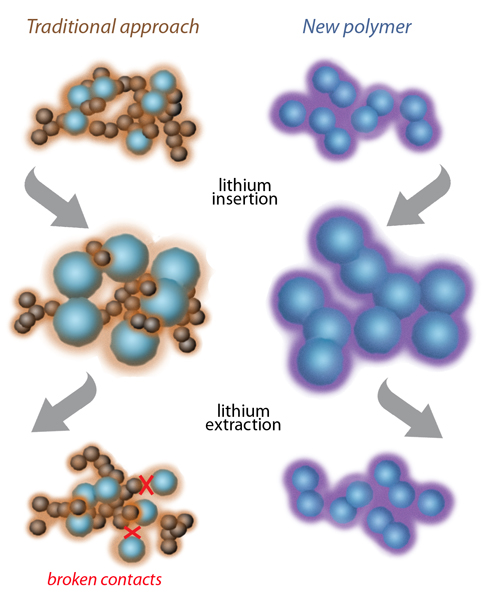
The secret is a tailored polymer that conducts electricity and binds closely to lithium-storing silicon particles, even as they expand to more than three times their volume during charging and then shrink again during discharge. The new anodes are made from low-cost materials, compatible with standard lithium-battery manufacturing technologies. The research team reports its findings in Advanced Materials, now available online.
High-capacity expansion
“High-capacity lithium-ion anode materials have always confronted the challenge of volume change — swelling — when electrodes absorb lithium,” says Gao Liu of Berkeley Lab’s Environmental Energy Technologies Division (EETD), a member of the BATT program (Batteries for Advanced Transportation Technologies) managed by the Lab and supported by DOE’s Office of Vehicle Technologies.
Says Liu, “Most of today’s lithium-ion batteries have anodes made of graphite, which is electrically conducting and expands only modestly when housing the ions between its graphene layers. Silicon can store 10 times more — it has by far the highest capacity among lithium-ion storage materials — but it swells to more than three times its volume when fully charged.”
This kind of swelling quickly breaks the electrical contacts in the anode, so researchers have concentrated on finding other ways to use silicon while maintaining anode conductivity. Many approaches have been proposed; some are prohibitively costly.
One less-expensive approach has been to mix silicon particles in a flexible polymer binder, with carbon black added to the mix to conduct electricity. Unfortunately, the repeated swelling and shrinking of the silicon particles as they acquire and release lithium ions eventually push away the added carbon particles. What’s needed is a flexible binder that can conduct electricity by itself, without the added carbon.
“Conducting polymers aren’t a new idea,” says Liu, “but previous efforts haven’t worked well, because they haven’t taken into account the severe reducing environment on the anode side of a lithium-ion battery, which renders most conducting polymers insulators.”
One such experimental polymer, called PAN (polyaniline), has positive charges; it starts out as a conductor but quickly loses conductivity. An ideal conducting polymer should readily acquire electrons, rendering it conducting in the anode’s reducing environment.
The signature of a promising polymer would be one with a low value of the state called the “lowest unoccupied molecular orbital,” where electrons can easily reside and move freely. Ideally, electrons would be acquired from the lithium atoms during the initial charging process. Liu and his postdoctoral fellow Shidi Xun in EETD designed a series of such polyfluorene-based conducting polymers — PFs for short.
When Liu discussed the excellent performance of the PFs with Wanli Yang of Berkeley Lab’s Advanced Light Source (ALS), a scientific collaboration emerged to understand the new materials. Yang suggested conducting soft x-ray absorption spectroscopy on Liu and Xun’s candidate polymers using ALS beamline 8.0.1 to determine their key electronic properties.
Says Yang, “Gao wanted to know where the ions and electrons are and where they move. Soft x-ray spectroscopy has the power to deliver exactly this kind of crucial information.”
Compared with the electronic structure of PAN, the absorption spectra Yang obtained for the PFs stood out immediately. The differences were greatest in PFs incorporating a carbon-oxygen functional group (carbonyl).
“We had the experimental evidence,” says Yang, “but to understand what we were seeing, and its relevance to the conductivity of the polymer, we needed a theoretical explanation, starting from first principles.” He asked Lin-Wang Wang of Berkeley Lab’s Materials Sciences Division (MSD) to join the research collaboration.
Wang and his postdoctoral fellow, Nenad Vukmirovic, conducted ab initio calculations of the promising polymers at the Lab’s National Energy Research Scientific Computing Center (NERSC). Wang says, “The calculation tells you what’s really going on — including precisely how the lithium ions attach to the polymer, and why the added carbonyl functional group improves the process. It was quite impressive that the calculations matched the experiments so beautifully.”
The simulation did indeed reveal “what’s really going on” with the type of PF that includes the carbonyl functional group, and showed why the system works so well. The lithium ions interact with the polymer first, and afterward bind to the silicon particles. When a lithium atom binds to the polymer through the carbonyl group, it gives its electron to the polymer — a doping process that significantly improves the polymer’s electrical conductivity, facilitating electron and ion transport to the silicon particles.







