Empa researchers have developed such a photoelectrochemical cell, recreating a moth’s eye to drastically increase its light collecting efficiency.
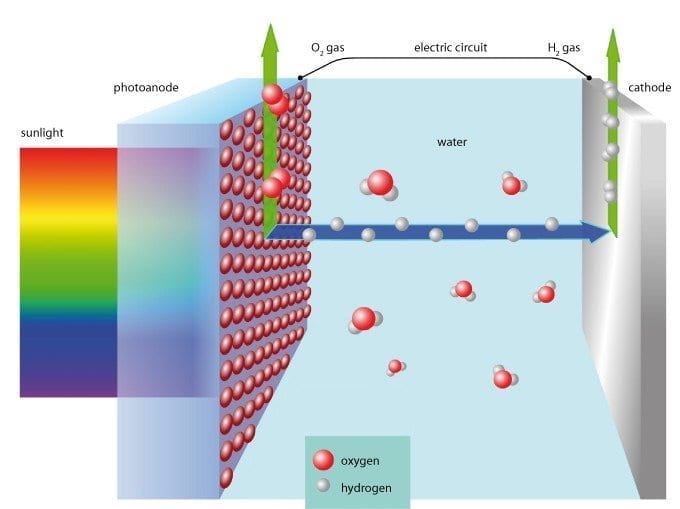
All over the world researchers are investigating solar cells which imitate plant photosynthesis, using sunlight and water to create synthetic fuels such as hydrogen. Empa researchers have developed such a photoelectrochemical cell, recreating a moth’s eye to drastically increase its light collecting efficiency. The cell is made of cheap raw materials – iron and tungsten oxide.
Rust – iron oxide – could revolutionise solar cell technology. This usually unwanted substance can be used to make photoelectrodes which split water and generate hydrogen. Sunlight is thereby directly converted into valuable fuel rather than first being used to generate electricity. Unfortunately, as a raw material iron oxide has its limitations. Although it is unbelievably cheap and absorbs light in exactly the wavelength region where the sun emits the most energy, it conducts electricity very poorly and must therefore be used in the form of an extremely thin film in order for the water splitting technique to work. The disadvantage of this is that these thin-films absorb too little of the sunlight shining on the cell.
Microspheres to collect the sunlight
Empa researchers Florent Boudoire and Artur Braun have now succeeded in solving this problem. A special microstructure on the photoelectrode surface literally gathers in sunlight and does not let it out again. The basis for this innovative structure are tiny particles of tungsten oxide which, because of their saturated yellow colour, can also be used for photoelectrodes. The yellow microspheres are applied to an electrode and then covered with an extremely thin nanoscale layer of iron oxide. When external light falls on the particle it is internally reflected back and forth, till finally all the light is absorbed. All the entire energy in the beam is now available to use for splitting the water molecules.
Read more . . .
The Latest on: Producing hydrogen with sunlight
[google_news title=”” keyword=”Producing hydrogen with sunlight” num_posts=”10″ blurb_length=”0″ show_thumb=”left”]
via Google News
The Latest on: Producing hydrogen with sunlight
- Shining a light on energy sustainability with synthetic methaneon May 1, 2024 at 10:02 pm
By producing synthetic methane using the sun, this team of researchers is working towards closing the carbon loop. Methane, the ...
- Green Hydrogen in Transportation: Fueling the Clean Mobility Revolutionon May 1, 2024 at 7:35 am
Green hydrogen is emerging as a clean fuel for transportation, promising to reduce emissions and revolutionize the energy sector.
- EU to provide €720 million to support hydrogen projectson May 1, 2024 at 5:25 am
The European Hydrogen Bank’s pilot auction is delivering €720 million ($768.3 million) to seven renewable hydrogen projects in Finland, Norway, Portugal and Spain. Together, they plan to produce 1.58 ...
- Nuclear Fusion Breakthroughs Bring Near-Limitless Energy Closeron April 30, 2024 at 5:46 am
Physicists say the discovery offers a potential avenue to "economically attractive fusion energy" in the future.
- Air Products CEO projects growth, willing to take some 'heat'on April 30, 2024 at 3:18 am
U. MACUNGIE TWP., Pa. -- Air Products reported second-quarter results Tuesday that were mixed, but Chief Executive Officer Seifi Ghasemi pointed to a steady increase in earnings and the company's ...
- Tribal company and Oregon battery startup create unique partnership in pursuit of clean energyon April 29, 2024 at 2:03 pm
Madisen McCleary, Skip Tech's vice president of engineering, makes a hydrophobic coating for the interior of a flow cell for the startup's hydrogen ...
- The top 10 Green Hydrogen initiatives to watch out foron April 26, 2024 at 5:56 am
In this article, we will explore the top 10 green hydrogen initiatives that are shaping the future of energy and sustainability. The top 10 Green Hydrogen initiatives to watch out for. Exploration & ...
- Green hydrogen in Nova Scotiaon April 26, 2024 at 2:10 am
Nova Scotia has announced its green hydrogen action plan, calling it an “alternative clean energy source” and adding that we’re emerging as a region with “ample opportunity” to produce the product — ...
- ‘Green hydrogen’ company looks to make Mississippi a leader of new renewable ventureon April 24, 2024 at 7:19 am
Hy Stor Energy will store its hydrogen in different salt domes around the state to feed a burgeoning renewable power source called "green hydrogen." ...
- Advanced Hydrogen Producing Equipment Wins Top Prize and $1 Million in TERA-Award Competitionon April 22, 2024 at 7:18 am
The results of the third TERA-Award Smart Energy Innovation Competition, hosted by Towngas, have been announced in Hong Kong. The Gold Award and a prize of US$1 million were won by a project of ...
via Bing News