A particularly ingenious remedy for the problem of rust may be available soon.
Containers made of a conductive polymer only open in the presence of corrosion and release anticorrosive payloads.
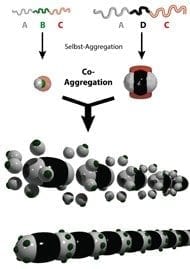
Scientists from the Max-Planck-Institut für Eisenforschung GmbH in Düsseldorf and the Max Planck Institute for Polymer Research in Mainz have succeeded in making two enormous strides towards developing a self-healing anticorrosion coating. In one study, they embedded a few 100-nanometre-sized polymer capsules containing anticorrosion payloads in a coating. They applied the coating to a metal and exposed the metal to corrosion through a crack in the coating. Thereupon, the capsules opened and released the protective payloads. As soon as the corrosive attack ended, the containers closed again. In the second study, the researchers encapsulated substances in nanocontainers that can heal small cracks and holes in the protective metal coating. The researchers thereby demonstrated that the containers were chemically altered and released the healing payloads when the corrosion process started. The containers then closed again at the end of the corrosive attack.
Human and animal skin is exemplary in many respects. Materials scientists are impressed above all by the way in which it heals itself when damaged. They would like to endow anticorrosion coatings with this very capacity, so that fine cracks and small holes in coatings do not spell disaster in the short or long term for the underlying metal. “We have made two breakthroughs in the quest for intelligent corrosion protection,” reports Michael Rohwerder, Leader of a Research Group at the Max-Planck-Institut für Eisenforschung.
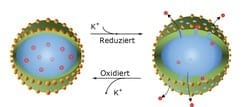
Together with their colleagues from the Max Planck Institute for Polymer Research, the Düsseldorf-based researchers tested capsules made from the conductive polymer polyaniline as containers for anticorrosive substances. They had decorated the nanocapsules with metal nanoparticles to generate suitable electrical contact between the containers and the metal to which they applied the capsules as components in a coating. Through a defect in the protective coating, they exposed the metal to corrosion by trickling a drop of salty water onto the opening in the protective coating. The corrosive attack, however, had no effect, as the walls of the polymer capsules became porous, allowing the substances contained in them to escape, which then blocked the oxygen reduction process.
The electrochemical potential is the most reliable key for opening the capsules
“What is crucial here is to select the correct signal for opening the capsule wall,” says Michael Rohwerder. The capsules can thus be opened purely mechanically when the protective coating is scratched. Or they can react to a rising pH value, which can accompany the process of corrosion. However, the Max Planck team opted to exploit the electrochemical potential as a capsule opener that punctured the polyaniline cover through a process of chemical conversion. “This potential always falls when corrosion starts,” explains Rohwerder. “So it provides the most reliable signal for the capsules to open.” Moreover, electrical contact is required for the capsules to recognise the electrochemical alarm as well. This is provided by the metal nanoparticles between the capsule wall and the metal. The capsules detect when the corrosion has stopped through the same information channel, as the potential rises constantly at this point. The capsule wall then restructures itself and the pores are re-sealed.
The containers, in which the researchers enclosed payloads can be used to form a polymer skin. These payloads can polymerise in a defect and seal the crack or hole. However, in this study the scientists did not apply the capsules to a metal using a coating to test them for corrosion. They replicated the chemical conditions that exist at the beginning and end of the corrosion process with reducing and oxidising substances and opened or closed the capsules in this way. “We were able to repeat this redox process with the polyaniline capsules over 80 times,” says Daniel Crespy, a Research Group Leader at the Max Planck Institute for Polymer Research, who supervised the study.
Go deeper with Bing News on:
Rust protection
- Rust-colored substance spreads in Lake Worth Lagoon near heralded manatee habitat
The source of a large rust-colored slick of goo that fouled the Intracoastal ... The Florida Department of Environmental Protection referred questions to the Coast Guard. Monday's unknown substance ...
- 6 Rust programming mistakes to watch out for
Rust offers programmers a way to write memory-safe software without garbage collection, running at machine-native speed. It's also a complex language to master, with a fairly steep initial ...
- Rust-Oleum 248657 Rubberized Undercoating Spray, Now 11% Off
For motorists that understand the importance of investing in high-quality vehicle protection, Rust-Oleum 248657 Rubberized Undercoating Spray should top your shopping list today. Available on Amazon, ...
- Rust Creek: 10 Similar Movies You Must See
Directed by Jen McGowan, ‘Rust Creek’ draws us into a gripping tale of survival set against the rugged backdrop of rural Kentucky. The film follows Sawyer, an intelligent college student who becomes ...
- Best Rust Converter & Remover: Options for Corrosion Protection
If you’ve ever faced years of rust and corrosion on metal parts, you know just how annoying it is to remove and how damaging it can be. Over time, rust can literally eat away at metal, leaving ...
Go deeper with Google Headlines on:
Rust protection
[google_news title=”” keyword=”Rust protection” num_posts=”5″ blurb_length=”0″ show_thumb=”left”]
Go deeper with Bing News on:
Self-healing anticorrosion coating
- Is self-healing code the next stage of GenAI?
Self-healing code refers to code that automatically corrects itself when issues occur, saving thousands of hours for developers who regularly test code for bugs and identify and correct them.
- Rediscovering Yourself: The 21-Day Reiki Self-Healing Journey
One powerful aspect of this practice is the self-healing component. For the curious and committed, the 21-day Reiki cleanse is a profound journey, bringing about a transformative self-awareness ...
- Marco Francesco D'Elia - ÁPEIROS
Corrosion is one of the most important causes of safety accidents and environmental hazards, which represent an extremely high economic burden for industry and ...
- Self-healing ceramic coatings: a game-changer for transportation
and is it possible to manufacture ceramic coatings with anti-corrosion and self-healing properties? Using a technique called Plasma Electrolytic Oxidation (PEO), Dr Mingo’s team is looking to achieve ...
- Self-Healing Concrete: What Ancient Roman Concrete Can Teach Us
Could so-called ‘hot mixing’, with pockets of reactive lime clasts inside the cured concrete provide self-healing properties? At its core, this is the recipe which any hydraulic cement uses ...
Go deeper with Google Headlines on:
Self-healing anticorrosion coating
[google_news title=”” keyword=”self-healing anticorrosion coating” num_posts=”5″ blurb_length=”0″ show_thumb=”left”]