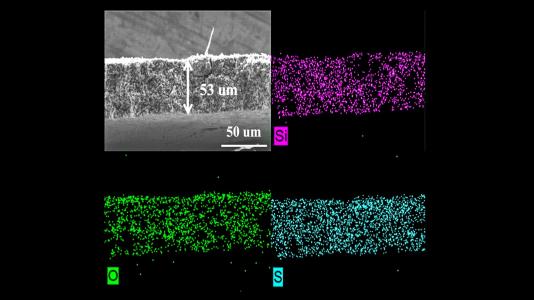
Image shows microstructure and elemental mapping (silicon, oxygen and sulfur) of porous sulfur-containing interlayer after 500 charge-discharge cycles in lithium-sulfur cell.
(Image by Guiliang Xu/Argonne National Laboratory.)
Batteries are everywhere in daily life, from cell phones and smart watches to the increasing number of electric vehicles. Most of these devices use well-known lithium-ion battery technology. And while lithium-ion batteries have come a long way since they were first introduced, they have some familiar drawbacks as well, such as short lifetimes, overheating and supply chain challenges for certain raw materials.
Scientists at the U.S. Department of Energy’s (DOE) Argonne National Laboratory are researching solutions to these issues by testing new materials in battery construction. One such material is sulfur. Sulfur is extremely abundant and cost effective and can hold more energy than traditional ion-based batteries.
In a new study, researchers advanced sulfur-based battery research by creating a layer within the battery that adds energy storage capacity while nearly eliminating a traditional problem with sulfur batteries that caused corrosion.
“These results demonstrate that a redox-active interlayer could have a huge impact on Li-S battery development. We’re one step closer to seeing this technology in our everyday lives.” — Wenqian Xu, a beamline scientist at APS
A promising battery design pairs a sulfur-containing positive electrode (cathode) with a lithium metal negative electrode (anode). In between those components is the electrolyte, or the substance that allows ions to pass between the two ends of the battery.
Early lithium-sulfur (Li-S) batteries did not perform well because sulfur species (polysulfides) dissolved into the electrolyte, causing its corrosion. This polysulfide shuttling effect negatively impacts battery life and lowers the number of times the battery can be recharged.
To prevent this polysulfide shuttling, previous researchers tried placing a redox-inactive interlayer between the cathode and anode. The term ?“redox-inactive” means the material does not undergo reactions like those in an electrode. But this protective interlayer is heavy and dense, reducing energy storage capacity per unit weight for the battery. It also does not adequately reduce shuttling. This has proved a major barrier to the commercialization of Li-S batteries.
To address this, researchers developed and tested a porous sulfur-containing interlayer. Tests in the laboratory showed initial capacity about three times higher in Li-S cells with this active, as opposed to inactive, interlayer. More impressively, the cells with the active interlayer maintained high capacity over 700 charge-discharge cycles.
“Previous experiments with cells having the redox-inactive layer only suppressed the shuttling, but in doing so, they sacrificed the energy for a given cell weight because the layer added extra weight,” said Guiliang Xu, an Argonne chemist and co-author of the paper. ?“By contrast, our redox-active layer adds to energy storage capacity and suppresses the shuttle effect.”
To further study the redox-active layer, the team conducted experiments at the 17-BM beamline of Argonne’s Advanced Photon Source (APS), a DOE Office of Science user facility. The data gathered from exposing cells with this layer to X-ray beams allowed the team to ascertain the interlayer’s benefits.
The data confirmed that a redox-active interlayer can reduce shuttling, reduce detrimental reactions within the battery and increase the battery’s capacity to hold more charge and last for more cycles. ?“These results demonstrate that a redox-active interlayer could have a huge impact on Li-S battery development,” said Wenqian Xu, a beamline scientist at APS. ?“We’re one step closer to seeing this technology in our everyday lives.”
Going forward, the team wants to evaluate the growth potential of the redox-active interlayer technology. ?“We want to try to make it much thinner, much lighter,” Guiliang Xu said.
Original Article: Lithium-sulfur batteries are one step closer to powering the future
More from: Argonne National Laboratory
The Latest Updates from Bing News
Go deeper with Bing News on:
Lithium-sulfur batteries
- More efficient molecular motor widens potential applications
Light-driven molecular motors were first developed nearly 25 years ago at the University of Groningen, the Netherlands. This resulted in a shared Nobel Prize for Chemistry for Professor Ben Feringa in ...
- Differentiating cerebral cortical neurons to decipher molecular mechanisms of neurodegeneration
A research team led by Professor Haruhisa Inoue (Department of Cell Growth and Differentiation) derived iPS cells (iPSC) from α-synucleinopathy patients with early-onset familial Parkinson's disease ...
- Nathan Derr
The Derr lab also pursues synthetic biology and the application of molecular motors to engineered nanoscale transport devices. ** = undergraduate co-authors Derr ND. Interactions of multiple dynein ...
- Molecular motors
Synthetic molecular motors represent a promising alternative approach to gain further insight into the principles by which biological motors function. Better understanding the mechanisms of ...
- Molecular crystal motors move like microbes when exposed to light
NEW ORLEANS, March 19, 2024 — At first glance, Rabih O. Al-Kaysi’s molecular motors look like the microscopic worms you’d see in a drop of pond water. But these wriggling ribbons are not alive; ...
Go deeper with Bing News on:
Li-S battery development
- Somers Officials Speak Out Against Battery Storage Facility
County and town officials in Westchester are speaking out against a proposed battery storage facility in the Hudson Valley that may pose safety concerns and has already garnered an opposing petition ...
- Lithium Miners News For The Month Of April 2024
Welcome to the April 2024 edition of the lithium miner news. The past month saw lithium prices flat and a very busy month of good news for the lithium producers. We also saw the start of the Beijing ...
- CATL unveils LFP battery with 621-mile range
The world’s biggest maker of EV batteries said it’s responding to drivers who still have anxiety about range and charging infrastructure.
- A framework to compare lithium battery testing data and results during operation
Reliably monitoring the amount of lithium (Li) present in rechargeable batteries, specifically in the so-called cathode active material (CAM), is key to understanding the condition of batteries from ...
- Olathe trash truck fire blamed on improperly disposed lithium battery
A spokesperson for the City of Olathe says a lithium ion battery is behind the fire that damaged one of their garbage trucks last week.