Researchers at the University of California San Diego and the Massachusetts Institute of Technology (MIT) have come up with a strategy for using synthetic biology in therapeutics. The approach enables continual production and release of drugs at disease sites in mice while simultaneously limiting the size, over time, of the populations of bacteria engineered to produce the drugs. The findings are published in the July 20 online issue of Nature.
UC San Diego researchers led by Jeff Hasty, a professor of bioengineering and biology, engineered a clinically relevant bacterium to produce cancer drugs and then self-destruct and release the drugs at the site of tumors. The team then transferred the bacterial therapy to their MIT collaborators for testing in an animal model of colorectal metastasis. The design of the therapy represents a culmination of four previous Nature papers from the UC San Diego group that describe the systematic development of engineered genetic clocks and synchronization. Over the years, the researchers have employed a broad approach that spans the scales of synthetic biology,
The new study offers a therapeutic approach that minimizes damage to surrounding cells.
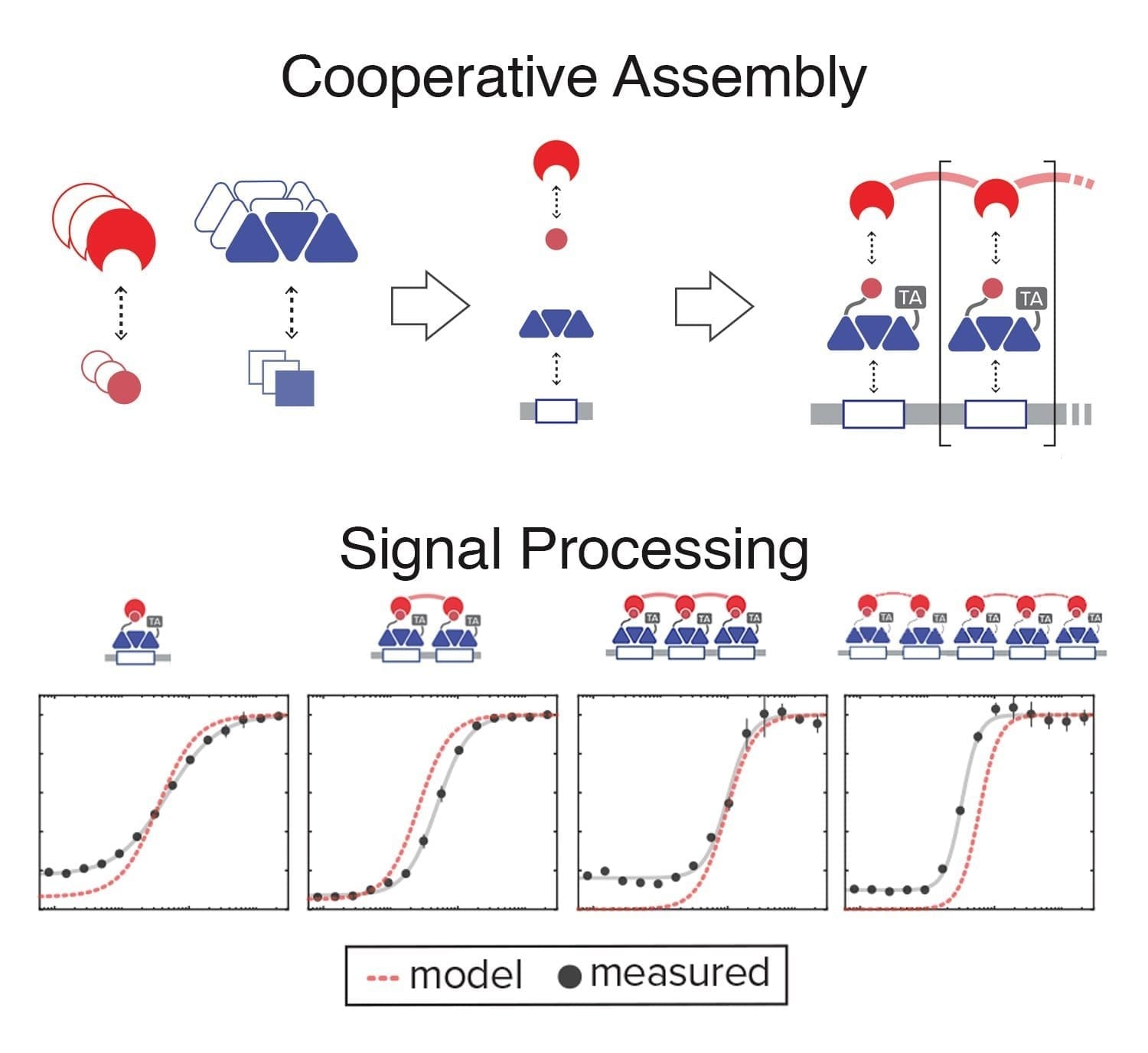
“In synthetic biology, one goal of therapeutics is to target disease sites and minimize damage,” said UC San Diego bioengineering and biology professor Jeff Hasty. He wondered if a genetic “kill” circuit could be engineered to control a population of bacteria in vivo, thus minimizing their growth. “We also wanted to deliver a significant therapeutic payload to the disease site.”
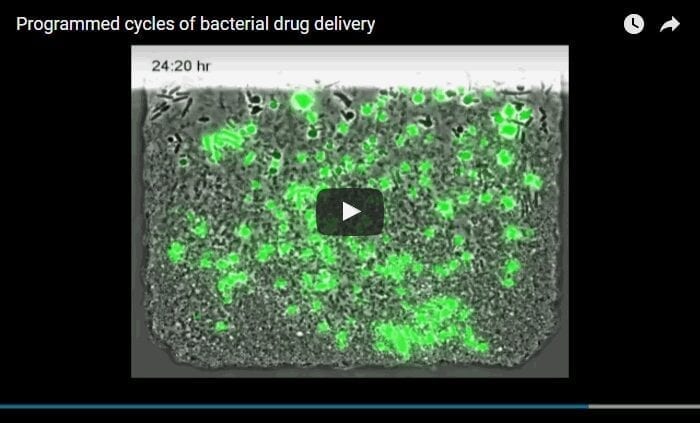
In order to achieve this, he and his team synchronized the bacteria to release bursts of known cancer drugs when a bacterial colony self-destructs within the tumor environment. The use of bacteria to deliver cancer drugs in vivo is enticing because conventional chemotherapy doesn’t always reach the inner regions of a tumor, but bacteria can colonize there. Importantly, the researchers observed that the combination of chemotherapy and the gene products produced by the bacterial circuit consistently reduced tumor size.
“The new work by Jeff Hasty and team is a brilliant demonstration of how theory in synthetic biology can lead to clinically meaningful advances,” said Jim Collins, a professor at MIT who is known as a founder of the field of synthetic biology. “Over a decade ago during the early days of the field, Jeff developed a theoretical framework for synchronizing cellular processes across a community of cells. Now his team has shown experimentally how one can harness such effects to create a novel, clinically viable therapeutic approach.”
Limiting the bacterial population
In order to observe the bacterial population dynamics, the researchers designed custom microfluidic devices for careful testing before investigations in animal disease models. Consistent with the engineering design, they observed cycling of the bacterial population that successfully limits overall growth while simultaneously enabling production and release of encoded cargo. When the bacteria were equipped with a gene that drives production of a therapeutic, the synchronized lysis of the bacterial colony was shown to kill human cancer cells. It is the first engineered gene circuit in synthetic biology to achieve these objectives.
Learn more: Synthetic Biology Used to Limit Bacterial Growth and Coordinate Drug Release
The Latest on: Synthetic biology
[google_news title=”” keyword=”synthetic biology” num_posts=”10″ blurb_length=”0″ show_thumb=”left”]
via Google News
The Latest on: Synthetic biology
- U of T researchers develop new approach for controlling neuron generation in Parkinson's diseaseon April 30, 2024 at 11:25 pm
Researchers at the University of Toronto have found a way to better control the preclinical generation of key neurons depleted in Parkinson's disease, pointing toward a new approach for a disease with ...
- First-batch alumna Jaya Deshmukh named new Mica directoron April 30, 2024 at 4:54 pm
Mica announces the appointment of Jaya Deshmukh as the new director, a first-batch alumna with a background in tech giants like Google and Microsoft. She is set to take charge in July, bringing ...
- Transcript: World Stage: Chinaon April 30, 2024 at 1:49 pm
Welcome to Washington Post Live. I’m David Ignatius, a columnist at The Post. Today I'm pleased to be joined by Dmitri Alperovitch, the co-founder of the cybersecurity firm CrowdStrike, co-founder of ...
- An AI model to reduce uncertainty in evapotranspiration predictionon April 30, 2024 at 12:37 pm
When scientists look at the Earth's available water for ecosystem services, they don't just look at precipitation. They must also account for water moving from the ground to the atmosphere, a process ...
- BioP2P’s Matt Gardner to Speak at SynBioBeta Global Synthetic Biology Conferenceon April 30, 2024 at 9:03 am
Matt Gardner, Board President of the California Biomanufacturing Center and Chair of the BioProcess to Product Network (BioP2P), is scheduled to speak at the 2024 SynBioBeta Global Biology Conference.
- Ansa Biotechnologies to Showcase Early Access Customer Success at Upcoming Global Synthetic Biology Conferenceon April 30, 2024 at 5:03 am
Ansa Biotechnologies, Inc., the trusted partner for complex DNA synthesis, today announced its speaker lineup for presentations at the upcoming Global Synthetic Biology (SynBioBeta) Conference in San ...
- Asimov achieves 10x improvement in lentiviral production, launches new stable cell line development serviceon April 30, 2024 at 12:04 am
Asimov, the synthetic biology company advancing the design and manufacture of therapeutics, today announced the expansion of its LV Edge System with the launch of a fully stable cell line ...
- ARPA-H leaders to participate in Johns Hopkins Health Policy Forum discussionon April 29, 2024 at 2:01 pm
School of Medicine Dean Theodore DeWeese will lead a conversation with Renee Wegrzyn, director of the Advanced Research Projects Agency for Health, and Kimberley Steele, ARPA-H program manager, on Apr ...
- Synthetic droplets cause a stir in the primordial soup: Chemotaxis research answers questions about biological movementon April 25, 2024 at 7:14 am
Simple yet profound questions like these are at the heart of curiosity-driven basic research, which focuses on the fundamental principles of natural phenomena. An important example is the process by ...
- “Truly Amazing” – Quantum Dots Successfully Synthesized Inside Living Cells!on April 24, 2024 at 11:43 pm
A recent study published in the journal National Science Review demonstrates the synthesis of quantum dots (QDs) in the nucleus of live cells. The research was conducted by Dr. Hu Yusi, Associate ...
via Bing News