The synthesis of ammonia, which is a raw material for chemical fertilizers and chemical products, uses more than 1% of the energy consumption of the world for its synthesis.
Therefore, this method of artificial photosynthesis that efficiently uses visible light can make a great contribution to energy savings on a global scale.
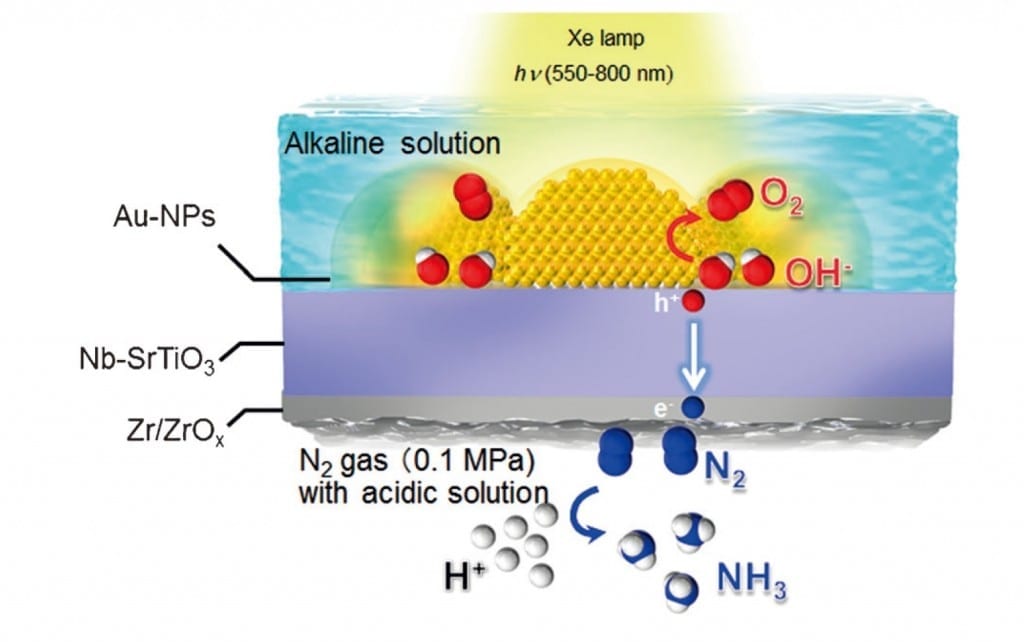
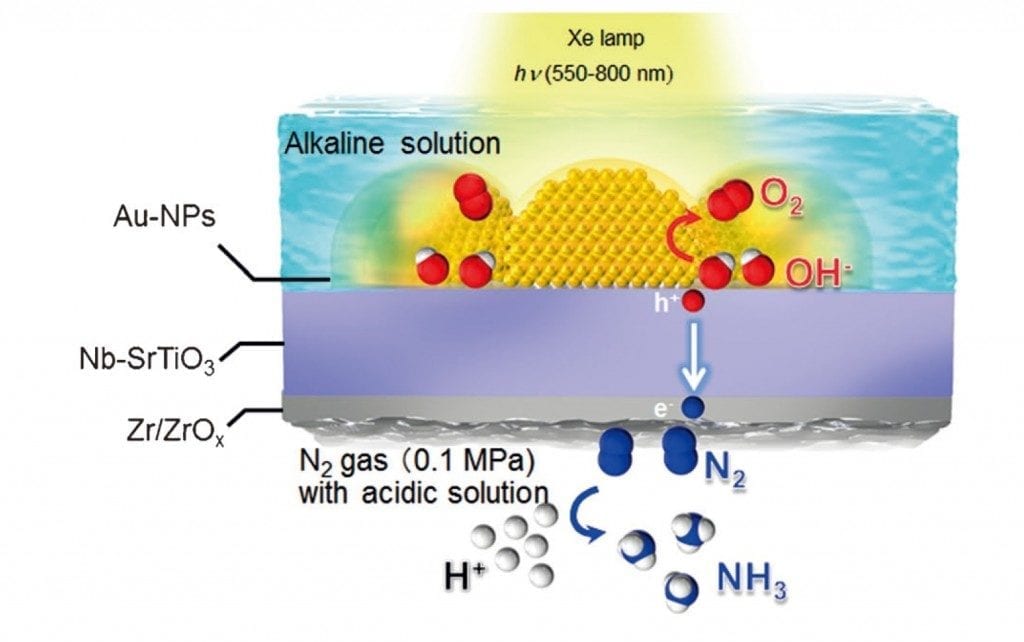
Ammonia has gained attention as a next-generation energy carrier. By combining an optical antenna structure that can concentrate light into a nano-space, and a co-catalyst that selectively adsorbs nitrogen, we have succeeded in selectively synthesizing ammonia from water and dinitrogen under visible light irradiation.
The research group of Professor Hiroaki Misawa and Assistant Professor Tomoya Oshikiri of the Research Institute for Electronic Science of Hokkaido University, by using a photoelectrode in which gold nanoparticles are loaded on an oxide semiconductor substrate, has worked to develop a method of artificial photosynthesis that has received attention as an ultimate light energy conversion system.
At this time this research group has developed a co-catalyst that can convert dinitrogen into ammonia with good efficiency, and by supporting this co-catalyst on a photoelectrode in which gold nanoparticles are loaded, they have succeeded in using water, dinitrogen, and visible light to selectively synthesize ammonia, which has gained attention as a next-generation energy carrier.
The research and development of artificial photosynthesis systems that convert solar energy into storable chemical energy have gained attention as a way to solve the global energy problem.
Ammonia is expected to be one of the next-generation energy carriers of chemical energy because it has little danger of combustion or explosion and can be liquefied relatively easily.
At the present time ammonia is commercially manufactured by the method called the Haber-Bosch process1, but this reaction requires a large amount of energy, and more than 1% of the world’s energy consumption goes into the Haber-Bosch process.
Accordingly, in order to efficiently use ammonia as an energy carrier, the world awaits the development of a low-energy synthesis method that is fundamentally different from conventional synthesis methods.
By combining an optical antenna structure that can concentrate light into a nano-space, and a co-catalyst that selectively adsorbs nitrogen, we have succeeded in synthesizing ammonia from nitrogen and water by using visible light, which is found in abundance in solar energy.
In the future, by improving the reaction efficiency and widening the response wavelength band, we will work towards the commercialization of this ultimate, clean “ammonia photosynthesis”, which can generate ammonia from the visible light in sunlight, the nitrogen in the air, and water.
Learn more: Successful Synthesis of Ammonia Using Visible Light, Water, and Atmospheric Nitrogen
The Latest on: Ammonia photosynthesis
[google_news title=”” keyword=”Ammonia photosynthesis” num_posts=”10″ blurb_length=”0″ show_thumb=”left”]
via Google News
The Latest on: Ammonia photosynthesis
- A Comprehensive Guide to Greenhouse Cleaningon April 24, 2024 at 9:00 am
Prepare your greenhouse for spring success! Unique tips for a thorough clean to optimize sunlight, thwart pests, and ensure healthy plant growth.
- Meet the boffins and buccaneers drilling for hydrogenon April 14, 2024 at 7:30 pm
Governments are throwing subsidies at efforts to make hydrogen fuel. America’s Inflation Reduction Act offers lavish support for producing it cleanly from fossil fuels (which involves the clunky ...
- Here's How Your Gorgeous Orchids Clean The Air In Your Homeon January 7, 2024 at 8:37 pm
In comparison, Cymbidium virescens was best at ridding the air of ammonia. On the other hand ... by taking in carbon dioxide and releasing oxygen into the atmosphere during photosynthesis. Although ...
- The Upside of Earth Day: Good News You Might Not Have Heard About Climate Change Actionon December 31, 2000 at 4:00 pm
Earth Day has been celebrated on April 22 since 1970. According to EarthDay.org, instead of seeing the holiday as just a stand-alone day each year, it Climate change remains a serious threat. However, ...
via Bing News