For many years, engineers have sought to create a special kind of surface: one that can both repel and absorb liquids, and whose ability to do so — its “wetting behavior” — can be quickly and precisely controlled. The technology would have a wide range of potential applications, from water filtration and biomedical devices to liquid optical lenses and lab-on-a-chip systems.
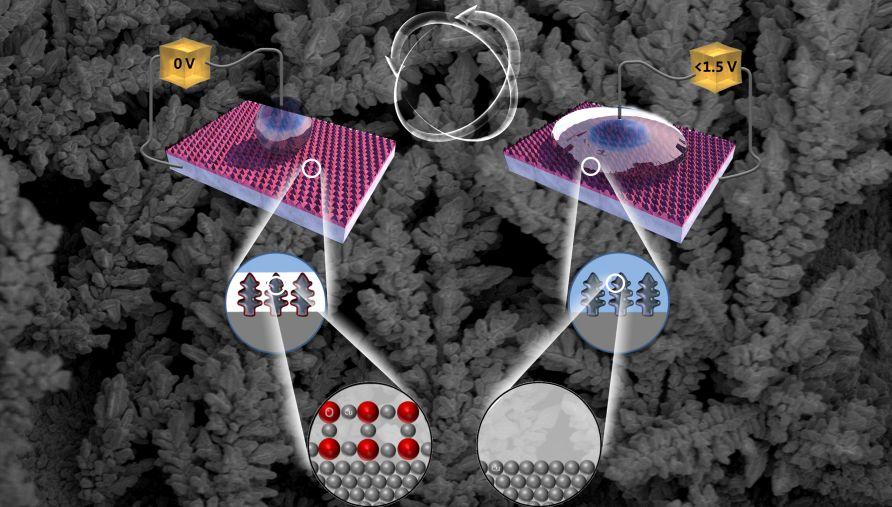
Such a “smart surface” has now been developed by researchers at the University of British Columbia. Inexpensive, scalable and powered by just a conventional electric battery, the copper-based surface changes from being very water-repellent (superhydrophobic) to very water-absorbent (superhydrophilic) as electric potential is applied.
“When tiny voltages are applied to the surface, water droplets that initially roll off stick to it more and more tightly,” says Ben Zahiri, the study’s co-lead author. “By changing the magnitude of the voltage and how long it is applied, we can easily control the angle that each droplet forms with the surface and how quickly this happens.”
When the electric potential is removed, the droplet retains its shape and remains pinned in place.
Other groups have modified the wetting behavior of copper surfaces using stimuli like heat, UV radiation and X-rays. But in order to achieve this, the required temperatures are high — up to 300 degrees Celsius — and the required exposure times are long — from tens of minutes to days. This makes them impractical for a number of consumer and industrial purposes.
In contrast, the electrical stimulus used by the UBC team modifies wetting behavior rapidly (from a few seconds to a few minutes) and reversibly, at voltages found in everyday batteries (less than 1.5 V). It does so by changing the oxidation state of the copper surface, which contains a mixture of hydrophilic CuO and hydrophobic Cu2O: as copper loses electrons, it becomes less attracted to water.
The ability to control surface wettability could be useful wherever droplets, or solid particles absorbed by droplets, need to be manipulated, including microfluidic devices and hazardous material handling systems. It also offers advanced self-cleaning capabilities by enabling the controlled roll-off of fluids.
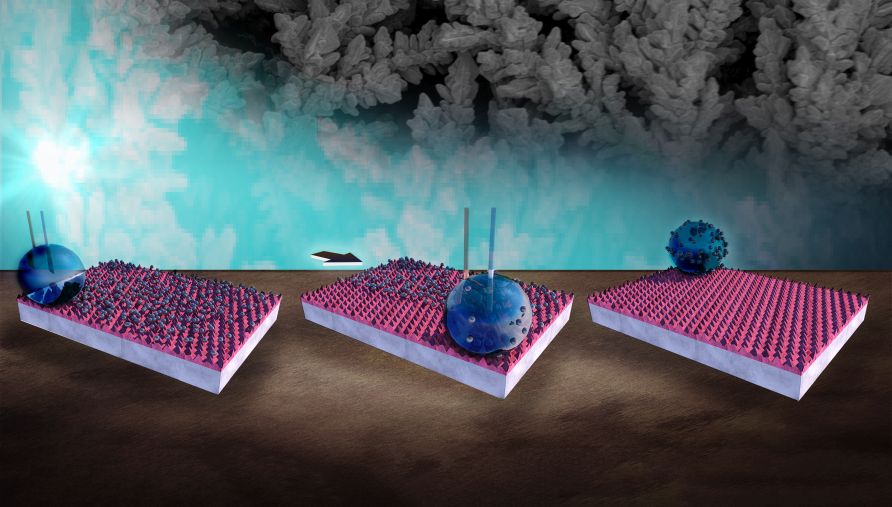
Although the UBC team chose to investigate copper because it is cheap, abundant and one of the most commonly used metals in the world, Zahiri believes that the electrochemical manipulation of other metals, metal oxides and mixed oxides may yield similarly promising results. As for the liquid, any conductive fluid, such as blood, could be used.
“These findings could open up a new area of exploration for smart surfaces,” says UBC mechanical engineering professor Walter Mérida, who supervised the work.
Learn more: SMART SURFACE ENABLES ADVANCED MANIPULATION OF DROPLETS
The Latest on: Smart surface
[google_news title=”” keyword=”smart surface” num_posts=”10″ blurb_length=”0″ show_thumb=”left”]- Going Green: Flood insurance is smart green thinkingon April 27, 2024 at 3:48 am
I’ve not been having the easiest time financially and have realised my flood insurance is really expensive, is it worth it? I’ve lived in my home for five years and never flooded?
- Nanoleaf x Umbra Cono Portable Smart Lamp review: a fun, versatile addition to any modern homeon April 26, 2024 at 4:21 am
One of the best things about the Nanoleaf x Umbra Cono is its versatile design. It can be positioned upright, on its side or upside down. Nanoleaf also mentions that its unique leg design means it can ...
- Mask-inspired perovskite smart windows enhance weather resistance and energy efficiencyon April 25, 2024 at 6:36 am
Thermochromic perovskite is a new color switch material used in energy-saving smart windows. Despite its potential for energy savings, thermochromic perovskite suffers from poor weather resistance, ...
- Surface Pro 10: all the major changes rumored for the new modelon April 23, 2024 at 12:39 pm
The Surface Pro 10 is rumored to be maing a big splash later this year.The Latest Tech News, Delivered to Your Inbox ...
- Xiaomi Smart Band 9: What you need to know about the next-gen wearableon April 23, 2024 at 5:00 am
The Xiaomi Smart Band 9 is expected to be the company’s next gen wearable product. The Chinese tech giant unveiled the Smart Band 8 back in 2023, and it appears that its successor is set to launch ...
- Green Builder Offers Solutions to Proposed Impervious Surface Code Changeon April 22, 2024 at 5:44 am
Proposed Dallas development code changes could limit impervious coverage for residential and nonresidential development.
- Are Utilities Getting Enough Value From Smart Meters?on April 22, 2024 at 3:30 am
Smart meter data, on the other hand, allows utilities to take a “bottom-up” approach to see “behind-the-meter” data from the individual home level. With the right analytics, this can even include ...
- Smart Solutions: Foldable furniture increases flexibilityon April 18, 2024 at 2:15 pm
From card tables and folding chairs to flexible desks and beds, these creative takes on standard styles work for entertaining as well.
- AI is making smart devices easier to hack—here's how to stay safeon April 17, 2024 at 8:55 am
From asking our smart speakers for the weather to receiving personalized advice from smartwatches, devices powered by artificial intelligence (AI) are increasingly streamlining our routines and ...
- Best Smart Speakers for 2024on April 15, 2024 at 3:01 pm
Smart speakers were popularized by Amazon’s Echo series of devices that were first unveiled back in 2014. At the time, the idea behind a smart speaker was to develop a device that would act as a smart ...
via Google News and Bing News