A major hurdle to creating a metal-free, 100-percent polymer battery is finding a polymer that is electrochemically active
Dr. Jodie L. Lutkenhaus, associate professor, holder of the William and Ruth Neely Faculty Fellowship and recently named Presidential Impact Fellow in the Artie McFerrin Department of Chemical Engineering at Texas A&M University, just published a paper in Nature Materials. The findings presented in this paper represent a significant step toward Lutkenhaus’s ultimate goal: the creation of a battery made entirely of polymers.
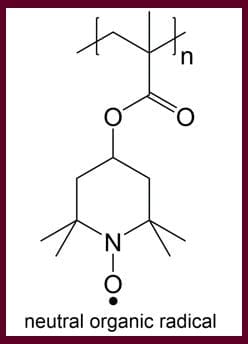
A major hurdle to creating a metal-free, 100-percent polymer battery is finding a polymer that is electrochemically active — meaning it has to be able to store and exchange electrons. Lutkenhaus, along with a team of researchers including doctoral candidate Shaoyang Wang, think that the organic radical polymers will do the trick. Owing to their chemical structure, organic radical polymers are very stable and reactive. As shown in the image below, organic radical polymers have a single electron on the radical group. This unpaired electron allows rapid charge transfer in these polymers during redox reactions.
According to Lutkenhaus, the main appeal of this class of polymer lies in the speed of the reaction. “These polymers are very promising for batteries because they can charge and discharge way faster than any common battery in a phone or similar device. This rapid charging could dramatically change the way electric vehicles are used today.”
The redox-active properties of organic radical polymers have been known for some time. However, prior to this research the exact mechanism by which electrons and ions are transported through the polymer had not been described. In part, the scale and speed at which these reactions take place make it difficult to capture reliable data. However, Lutkenhaus and her team were able to capture incredibly detailed measurements using a specialized device, an electrochemical quartz crystal microbalance with dissipation monitoring (EQCM-D).
The use of an EQCM-D is actually quite simple, but it operates on tremendously small scales. Lutkenhaus explained the experimental setup: “As we charge and discharge the polymer we are actually weighing it, so we know exactly how much it weighs even down to nanogram accuracy. The device is so sensitive that we can measure ions going in and out of the organic radical polymer.”
The results of the EQCM-D analysis led to somewhat unexpected results. Before this research the consensus was that only anions were transported in this process. However, the results show that lithium ions are transported as well. Further, the behavior and transport of the ions seems to be more dependent on the electrolyte than the polymer itself.
With this deeper understanding of the underlying processes, Lutkenhaus plans to take a closer look at the electrolyte polymer interactions.
Learn more: The energy implications of organic radical polymers
The Latest on: Polymer battery
[google_news title=”” keyword=”polymer battery” num_posts=”10″ blurb_length=”0″ show_thumb=”left”]
via Google News
The Latest on: Polymer battery
- Korean-German Partnership Accelerates Battery and Semiconductor Innovationson April 26, 2024 at 2:54 am
A collaboration program funded by the Korean Ministry of Trade, Industry and Energy (MOTIE) aims to expedite advancements in battery cell and sem ...
- Osmosis breakthrough: New battery uses river salt to generate electricityon April 25, 2024 at 6:24 am
Researchers have created a semipermeable membrane that generates electricity by absorbing osmotic energy from salt gradients. The advanced membrane significantly boosted the osmotic energy extracted ...
- Review: Lone Wolf's Alphawolf Caliber-Conversion Slideson April 25, 2024 at 3:38 am
For owners of .40 S&W-chambered Glocks, Lone Wolf's Alphawolf caliber-conversion slides are an easy way to update the design.
- This salt battery harvests osmotic energy where the river meets the seaon April 24, 2024 at 10:55 am
Estuaries -- where freshwater rivers meet the salty sea -- are great locations for birdwatching and kayaking. In these areas, waters containing different salt concentrations mix and may be sources of ...
- Revolutionizing Renewable Energy: Innovative Salt Battery Efficiently Harvests Osmotic Poweron April 24, 2024 at 5:00 am
A new semipermeable membrane doubles the osmotic energy output in estuaries, showing potential for sustainable power generation. Estuaries — where freshwater rivers meet the salty sea — are great ...
- Aviation-specific battery system uses advanced composites to address electric, hybrid flighton April 23, 2024 at 10:23 pm
BOLDair’s composite enclosures, compression structures and thermal runaway management enables high-performance electric energy storage.
- Mophie Juice Pack for the iPhone 15 is Now Available on Zagg.com!on April 23, 2024 at 3:41 pm
mophie, a leading brand in power and innovator of intelligent solutions for mobile devices, today announced the return of the iconic juice pack cellphone ...
- Advanced Batteries and Sustainability - IDTechEx Experts Discuss Battery Technologieson April 23, 2024 at 4:02 am
Battery innovation underpins many sectors, including electric vehicles, sustainability, and renewable energy. In a recent episode of their technology podcast ' Tomorrow's Tech with IDTechEx ', ...
- Innovative nanocoating shown to significantly enhance battery casing fire resistanceon April 23, 2024 at 1:58 am
Science X is a network of high quality websites with most complete and comprehensive daily coverage of the full sweep of science, technology, and medicine news ...
- Preventing thermal runaway at every level of EV battery assemblyon April 21, 2024 at 3:07 am
There is currently a lack of clear regulation around TRP standards. But across the industry, many OEMs have adopted the “5-minute rule” as the standard, meaning if a thermal runaway event happens, ...
via Bing News