Scientists have developed a new chimeric antibody that suppresses malignant cancers in dogs, showing promise for safe and effective treatment of intractable cancers.
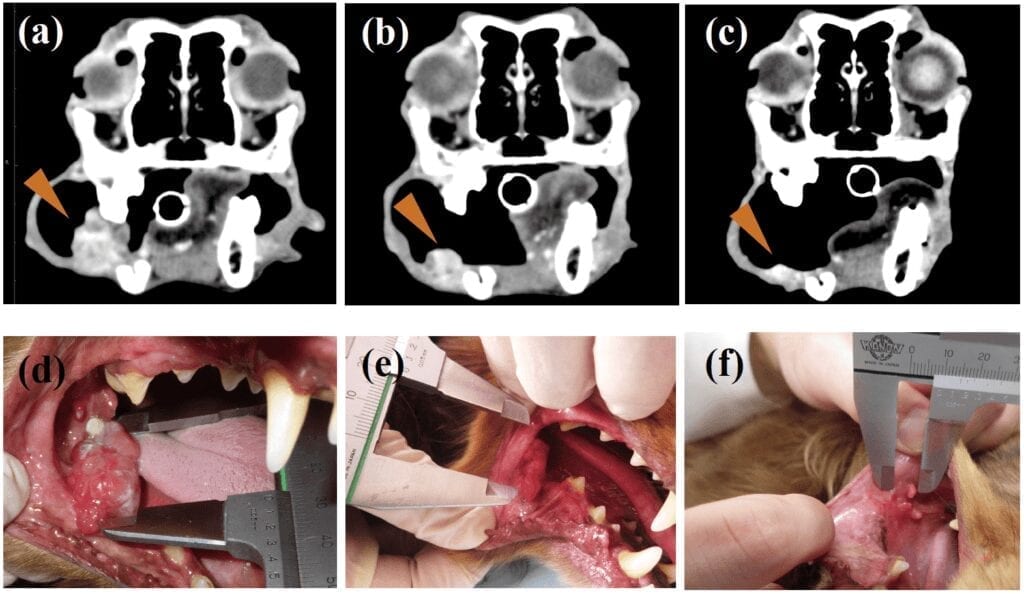
Similar to our aging society, dogs live longer than before and an increasing number of them die from cancer nowadays. As seen in humans, dogs have malignant cancers that cannot be treated by existing therapies such as surgery, radiotherapy and chemotherapy. Oral malignant melanoma (OMM), a highly invasive cancer in dogs, is one such example.
In humans, some malignant cancer cells express PD-L1 proteins that bind to their receptor PD-1 on T cells, resulting in the suppression of the T cell’s immune function. Thus, PD-L1/PD-1 interaction is considered an “immune escape mechanism” that cancer cells have. Antibodies that block PD-1/PD-L1 binding have proven effective in inducing anti-tumor immune responses and have been widely used in immunotherapy in the last five years. However, in dogs, no such clinical studies have been reported so far.
Professor Satoru Konnai of Hokkaido University and his collaborators in Japan have developed a chimeric anti-PD-L1 antibody that induces immune responses and therefore tumor regression in dogs with malignant cancers.
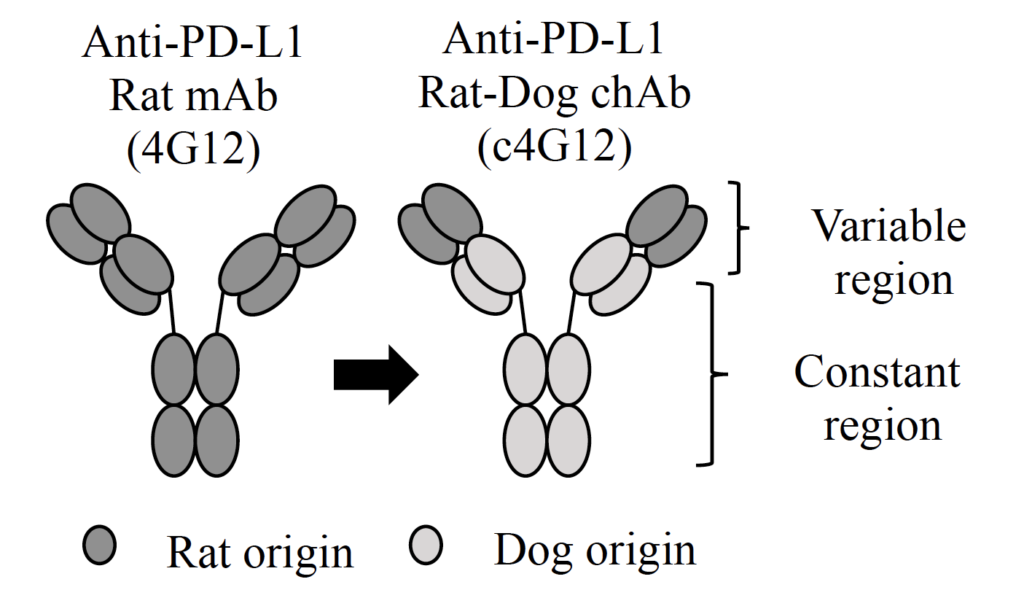
The team first revealed that PD-L1 is expressed in the cells of OMM and another type of cancer called undifferentiated sarcoma, confirming that those two cancers are likely targeted by the immunotherapy. They then utilized a rat anti-PD-L1 antibody to develop a rat-dog chimeric antibody which should help avoid rejection by the immune system and allergic reactions when administered to dogs.
In their pilot clinical study, seven dogs with OMM and two dogs with undifferentiated sarcoma were treated with the chimeric antibody every two weeks. One of the OMM dogs showed obvious tumor regression after ten weeks of administration while one dog with undifferentiated sarcoma showed a significant decrease in tumor burden after three weeks. None of them showed adverse effects such as an allergic reaction. Moreover, their data suggested the treatment may have prolonged survival in dogs with OMM after pulmonary metastasis.
“Chimerization of the antibody is now proven as a simple and effective strategy to develop therapeutic antibodies in veterinary medicine. Although further clinical studies are needed, other PD-L1-positive cancers could be targeted by the antibody we have developed,” says Satoru Konnai. “Given the similarity between humans and dogs in cancer biology, our study should provide a beneficial model for human preclinical studies.”
Learn more: New therapeutic antibody for dog cancers
The Latest on: Chimeric antibody
[google_news title=”” keyword=”chimeric antibody” num_posts=”10″ blurb_length=”0″ show_thumb=”left”]- Santen Pharmaceutical gets grant for monoclonal antibody with high affinity for VEGFon April 30, 2024 at 5:44 am
Discover how Santen Pharmaceutical's monoclonal antibody targeting VEGF offers groundbreaking advancements in treating eye diseases and cancers.
- Vaccinex gets grant for antibodies binding human CCR8 for treating diseaseson April 30, 2024 at 5:41 am
Discover how Vaccinex Inc's newly granted patent for antibodies targeting human CCR8 can revolutionize disease treatment. Learn about the specific amino acid sequences and therapeutic applications ...
- Immunopathogenesis of Allergic Disorders: Current Conceptson April 24, 2024 at 5:00 pm
neuromuscular blocking agents and foreign proteins (chimeric antibodies). In non-IgE-mediated drug allergy, different mechanisms are involved. These include cytotoxic/cytolytic reactions involving ...
- Chinese Medical Journal review article highlights the potential and promise of CAR-T cell therapy in autoimmune diseaseson April 22, 2024 at 3:47 pm
Researchers conduct bibliometric analysis to evaluate the use of chimeric antigen receptor ... B and T immune cells and autoantibodies—antibodies against body’s own proteins—may present ...
- Cancer Monoclonal Antibodies Market Size, Share, Growth Statistics, Latest Trends, and Forecast to 2024 to 2032on April 18, 2024 at 10:15 pm
Request To Download Free Sample of This Strategic Report @- Cancer Monoclonal Antibodies Market is valued at approximately USD 41.3 billion in 2019 and is anticipated to grow with a healthy growth ...
- Monoclonal antibodies in the clinicon March 28, 2024 at 1:37 am
Chimeric mAbs were less evident ... this class of mAbs has been the main source of antibody-based products entering clinical studies since 1997. Figure 1: Number of mAbs entering clinical studies ...
- Astellas secures approval for gastric cancer antibody in Japanon March 26, 2024 at 5:33 pm
Astellas' monoclonal antibody Vyloy (zolbetuximab) has secured approval from Japan’s Ministry of Health, Labour, and Welfare (MHLW) in combination with chemotherapy for the treatment of gastric ...
- Antibodies against anything? AI tool adapted to make themon March 20, 2024 at 8:26 am
Antibodies are incredibly useful. Lots of recently developed drugs rely on antibodies that bind to and block the activity of specific proteins. They're also great research tools, allowing us to ...
- IVBIY Innovent Biologics, Inc.on March 2, 2024 at 9:12 am
a fully-human anti-VEGF monoclonal antibody; HALPRYZA, a recombinant chimeric murine/human anti-CD20 monoclonal antibody; SULINNO, a fully-human antiTNF-a monoclonal antibody; Pemazyre ...
- Recombinant antibody expression services and a novel antibody generation platformon November 13, 2023 at 3:55 am
The production capabilities encompass a broad spectrum of antibody formats, including full-length, scFv, Fab, sdAb/VHH, Fc fusion proteins, chimeric antibodies, and bispecific antibodies.
via Google News and Bing News