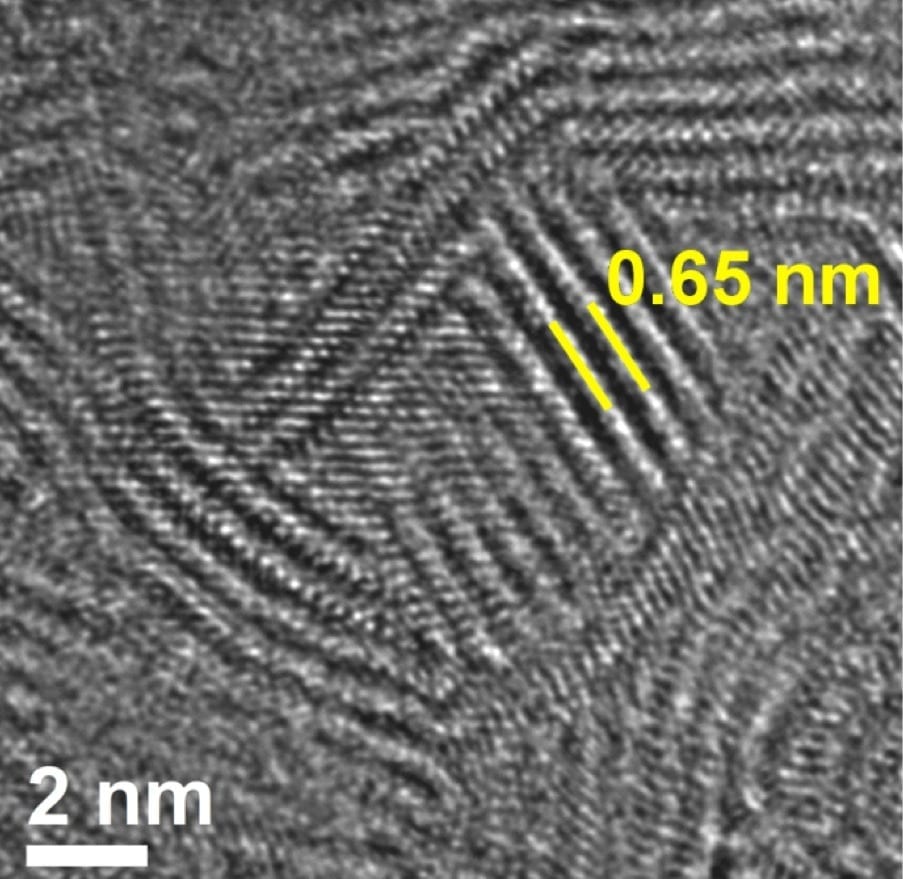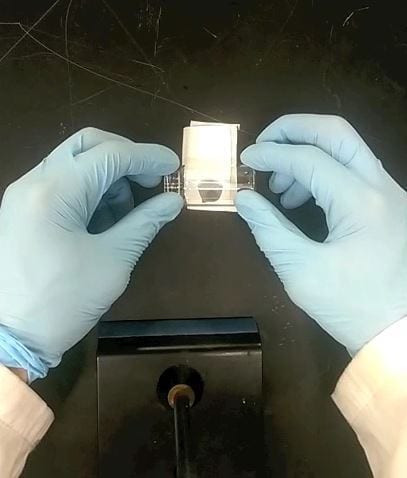
MXene clay, invented by researchers at Drexel University, can be rolled into any thickness or formed into any shape while retaining its electrical conductivity.
In the race to find materials of ever increasing thinness, surface area and conductivity to make better performing battery electrodes, a lump of clay might have just taken the lead.
Materials scientists from Drexel University’s College of Engineering invented the clay, which is both highly conductive and can easily be molded into a variety of shapes and sizes. It represents a turn away from the rather complicated and costly processing—currently used to make materials for lithium-ion batteries and supercapacitors—and toward one that looks a bit like rolling out cookie dough with results that are even sweeter from an energy storage standpoint.
With the publication of their recipe for “conductive MXene clay” in the Dec. 4 edition of Nature, the researchers suggest a significant shift in the way electrodes for storage devices are produced.
The clay, which already exhibits conductivity on par with that of metals, can be turned into a film—usable in an electrode—simply by rolling or pressing it.
“Both the physical properties of the clay, consisting of two-dimensional titanium carbide particles, as well as its performance characteristics, seem to make it an exceptionally viable candidate for use in energy storage devices like batteries and supercapacitors,” said Yury Gogotsi, PhD, Distinguished University and Trustee Chair professor in the College of Engineering, and director of the A.J. Drexel Nanomaterials Institute, who is a co-author of the paper. “The procedure to make the clay also uses much safer, readily available ingredients than the ones we used to produce MXene electrodes in the past.”
The key to the utility of this material, according to Michel Barsoum, PhD, Distinguished professor in the College of Engineering and one of the inventors of MXenes, is in its form.
“As anybody who has played with mud can attest, clay is hydrophilic –water-loving,” Barsoum said. “Clay is also layered and when hydrated, the water molecules slide between the layers and render it plastic that in turn can be readily shaped into complex shapes. The same happens here; when we add water to MXene, water penetrates between the layers and endows the resulting material with plasticity and moldability. Graphene—a material widely studied for use in electrodes- on the other hand, is conductive but does not like water—it is hydrophobic. What we discovered is a conductive two-dimensional layered material that also loves water. The fact that we can now roll our electrodes rapidly and efficiently, and not have to use binders and/or conductive additives renders this material quite attractive from a mass production point of view.”
The Latest on: Energy storage
[google_news title=”” keyword=”Energy storage” num_posts=”10″ blurb_length=”0″ show_thumb=”left”]
via Google News
The Latest on: Energy storage
- Texas energy storage dash brings 1 GW batteries within sighton May 9, 2024 at 9:27 am
Developers are installing larger batteries in Texas, with or without solar, capitalising on cost savings to maximise power revenues.
- A Staggering 19x Energy Jump in Capacitors May Be the Beginning of the End for Batterieson May 9, 2024 at 8:57 am
While batteries excel in storage capacity, they fall short in speed, unable to charge or discharge rapidly. Capacitors fill this gap, delivering the quick energy bursts that power-intensive devices ...
- Microgrid Sector Firms Working Together on 150-MWh Battery Storage Project in NYCon May 9, 2024 at 7:05 am
Eaton is working with fellow microgrid and on-site power developer Endurant Energy to deploy 10 battery storage projects that are planned to help strengthen grid reliability ...
- National Labs Guide Critical AI, Energy Storage, And Grid Researchon May 9, 2024 at 6:00 am
Artificial intelligence and other technologies will take energy production and deliverty to a new level, helping increase reliability, reduce emissions, and cut costs.
- Companies ink major deal on energy storage system: 'To ensure a stable and reliable energy grid'on May 9, 2024 at 3:30 am
Because the battery system will store this energy from wind and solar power, it will also be clean, making it safer and more sustainable. Companies ink major deal on energy storage system: 'To ensure ...
- Azerbaijan Explores Cooperation With Chinese Firms To Boost Renewable Energy Storageon May 9, 2024 at 3:08 am
The Azerbaijan Renewable Energy Agency (AREA), a key player inthe nation's energy sector, is actively seeking collaboration withChinese enterprises to enhance the utilization of battery andpumped ...
- Dragonfly Energy Announces Breakthrough in Lithium Battery Production: Eliminating Harmful “Forever Chemicals”on May 9, 2024 at 12:00 am
(“Dragonfly Energy” or the “Company”), an industry leader in green energy storage, has made a significant breakthrough in battery manufacturing with the successful production of PFAS-free electrodes ...
- New Pennsylvania PUC policy sparks debate over who can own energy storage assetson May 8, 2024 at 10:20 pm
Pennsylvania’s Public Utility Commission released a policy statement last month that included a definition of energy storage assets but took a neutral stance on their ownership. Utility companies ...
- Co-location of solar PV and energy storage: A key trend shaping the future of solar energy in SEEon May 8, 2024 at 5:00 pm
In SEE, the grids yearn for deep reinforcement to accommodate the burgeoning solar capacity. In the face of slow grid development, energy storage and flexible demand are stepping up as crucial ...
- Proposed Wheatland energy storage project could supply town with $4M in revenue over 20 yearson May 6, 2024 at 9:00 am
An Omaha, Neb.-based power development company is looking to build a 200-megawatt battery storage system in the Town of Wheatland.
via Bing News