Rice University scientists prepare elusive organocatalysts for drug and fine chemical synthesis
Rice University scientists using an efficient metal-free process have synthesized dozens of small-molecule catalysts, tools that promise to speed the making of novel chemicals, including drugs.
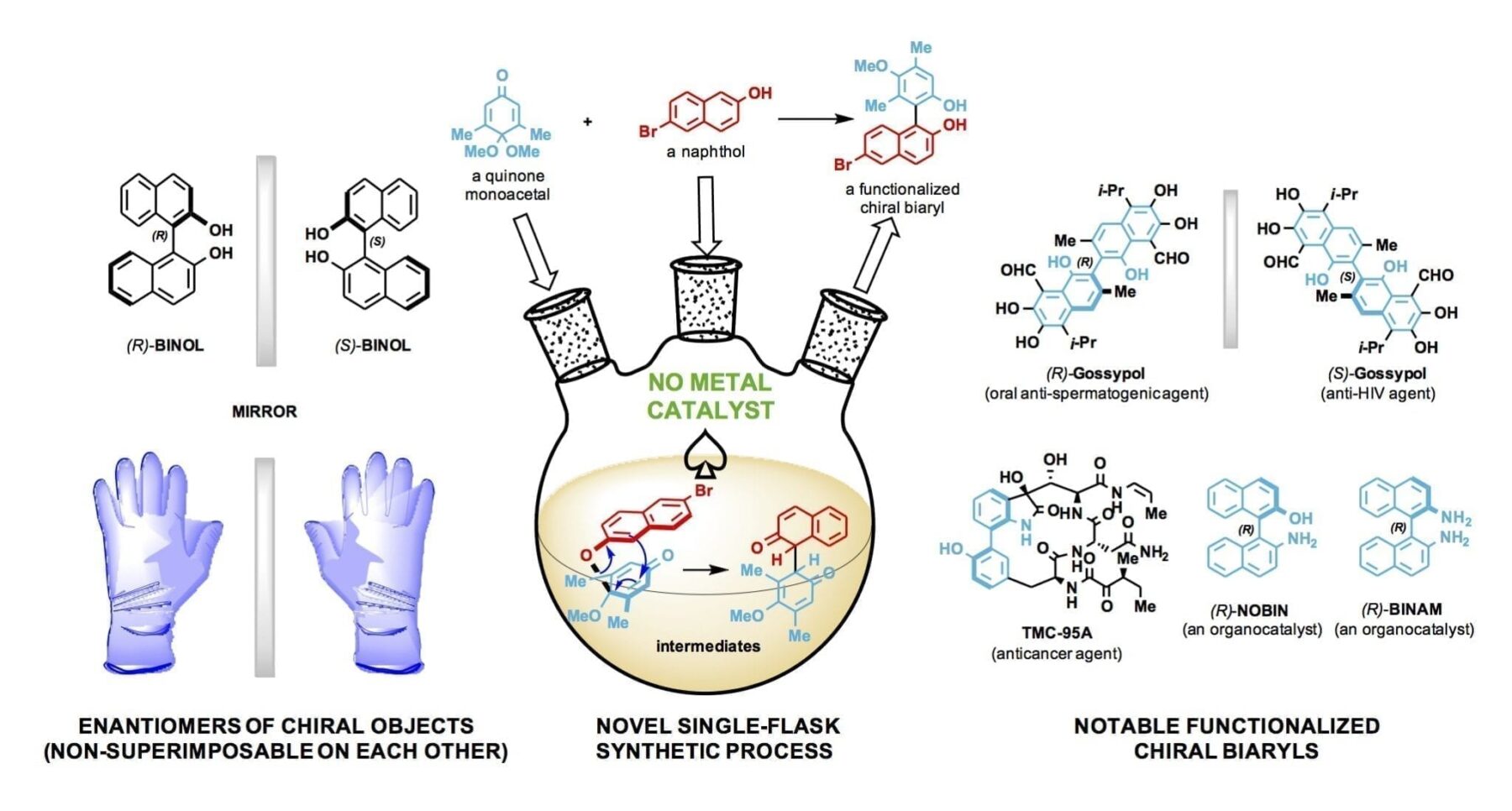
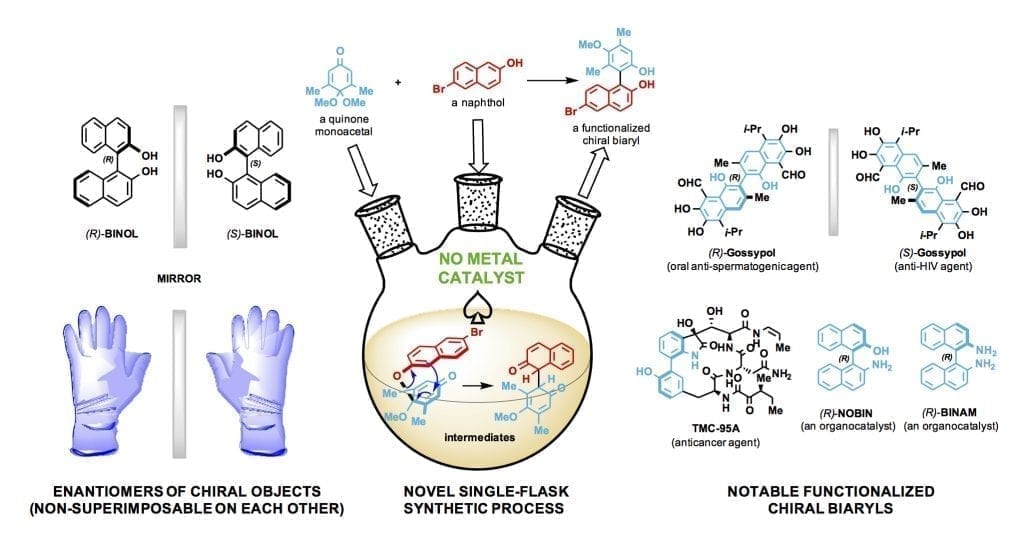
The lab of synthetic chemist László Kürti made elusive chiral biaryl compounds in a single-flask process that does not require the use of transition metals.
These biaryls are called organocatalysts because they catalyze chemical reactions without metal ions. They eliminate the need for transition metals and simplify chemical processes to synthesize new molecules. Transition metals are conductive metals that include titanium, iron, nickel, silver, copper, palladium and gold and are commonly used in catalysis.
The new tools detailed in the Angewandte Chemie international edition open avenues for faster and more cost-effective chemical synthesis, Kürti said.
Biaryls are molecular compounds of two aromatic rings directly joined by a carbon-carbon bond. When functionalized, or altered, these biaryls (phenyl-phenyl, naphthyl-phenyl, thienyl-naphthyl and more) become highly selective, reliable and customizable catalysts, Kürti said.
Kürti’s research uses biaryls as catalysts to develop novel single-enantiomer compounds.
Enantiomers are asymmetrical molecules found among organic compounds. Like one’s hands, their structures are mirror images that cannot be superimposed on each other. Significantly, these twins can have radically different effects – one beneficial, one not — as they interact with enzymes, proteins, receptors and even other chiral catalysts. Pharmaceutical companies understandably want to make drugs that contain only the helpful enantiomer.
Currently, single-enantiomer compounds are painstakingly synthesized as building blocks for drugs, agricultural products and functional materials.
Kürti said that by decade’s end, 95 percent of chiral drugs will be sold as single enantiomers. But synthesizing one particular enantiomer with precision and high efficiency is hard, especially via trial-and-error approaches that to now often require transition metal catalysts.
“For enantiomer preparations, you need catalysts,” he said, but transition metals are expensive and can leave toxic residues that need to be removed before the compound can be used in clinical trials. The Rice lab’s simple, cost-effective way to make chiral-functionalized biaryls not only eliminates the need for transition metals but can replace many steps in the synthesis process. Each step can take days or weeks.
Read more: Chemical design made easier
The Latest on: Chemical design
[google_news title=”” keyword=”Chemical design” num_posts=”10″ blurb_length=”0″ show_thumb=”left”]
via Google News
The Latest on: Chemical design
- Researchers repurpose commonplace chemical with incredible properties in new battery design: 'Exhibited remarkable cycling stability'on May 2, 2024 at 4:15 am
Scientists have created a new type of battery for grid energy storage by repurposing a chemical commonly used in water treatment plants. They say it has huge potential to increase grid resiliency.
- Classical challenges in the physical chemistry of polymer networkson April 30, 2024 at 5:00 pm
The design of polymer networks is one of the oldest and most important challenges in chemistry, impacting many of the highest volume chemical industries from rubber to adhesives to biomedical ...
- Scientists design super-battery made with cheap, readily affordable chemical element, Na — Salt-based cell has surprisingly good energy density and charges in secondson April 29, 2024 at 9:50 pm
Use precise geolocation data and actively scan device characteristics for identification. This is done to store and access information on a device and to provide personalised ads and content, ad and ...
- Chemical Engineeringon April 29, 2024 at 5:00 pm
Nuclear engineers design, build and develop the processes, instruments and nuclear systems that harness nuclear energy and radiation for the benefit of society and the environment. Our nuclear ...
- Chemical Engineering Design and Analysison April 27, 2024 at 10:54 am
This 1998 text offers a well-paced introduction to chemical engineering. Students are first introduced to the fundamental steps in design and three methods of analysis: mathematical modeling, ...
- Vilya Announces New Publication in Science Validating Foundational Approach to Design of Novel Chemically Diverse Macrocycleson April 25, 2024 at 1:00 pm
Company creating de novo designed macrocycles with enhanced efficacy that precisely target disease biology– Vilya’s privileged macrocycles have ...
- Chemical Engineeringon September 24, 2022 at 6:22 pm
Biology also plays an increasingly important role. What do chemical engineers do? Broadly, chemical engineers conceive and design processes involved in chemical manufacturing. The main role of ...
- Chemical Fume Hoodson February 25, 2022 at 7:08 am
Understanding the limitations, the appropriate maintenance techniques, and overall design of the fume hood will ensure your safety while using hazardous materials. The purpose of a chemical fume hood ...
- Senior Designon July 20, 2021 at 3:52 am
A major component of the chemical engineering undergraduate curriculum at Michigan Tech is the Senior Design experience, which occurs during the student’s senior year. Senior Design is six credits and ...
- Department of Chemical and Biological Engineeringon May 20, 2021 at 2:01 am
Chemical Engineering applies principles of math, chemistry, physics, and biology to the design of processes and products that advance technologies and solve problems touching every part of our lives.
via Bing News