Battery researchers at the Department of Energy Technology (IFE) have solved a challenge scientists worldwide are struggling with. It allows for far better batteries with higher capacity, without exceeding life.
– You can say we have found the x factor we’ve been looking for. This has enormous potential and is something scientists around the world are trying to make, says research director Arve Holt.
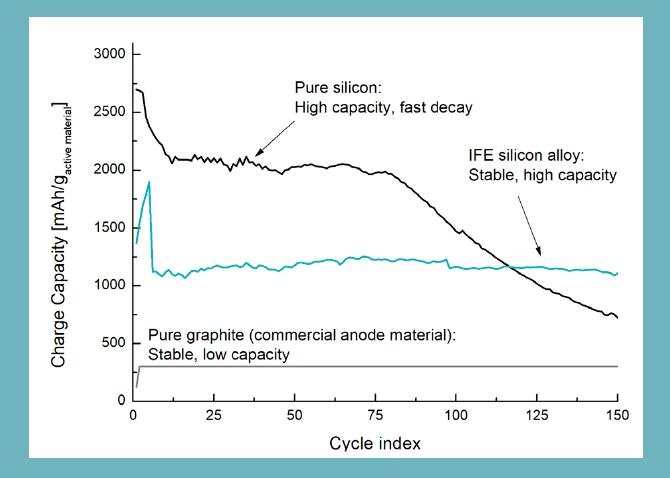
The research results show that with the new IFE-developed technology, it can achieve three to five times the charge capacity of the negative electrode (anode) as with today’s common graphite technology. Along with the developments seen in other parts of the battery, it may mean mobile phones that do not need to be charged on days or electric cars with 1000km range.
IFE is now ready to take the research into the market and is working on patenting the technology. The Institute will work in parallel with several Norwegian and international companies to thoroughly test the new battery.
“We have tested that it works on a lab scale with good results. Now that we have received support from the Research Council in the FORNY2020 program, we will test it further with international industry partners and see if it works in their industrial processes. The project, which will focus on bringing the new material to the market, we have called SiliconX, and it is very exciting to work towards such major goals together with Kjeller Innovation, says Marte O. Skare, one of the researchers in the project.
Silicon code
There are around 20 researchers working on silicon-related research at IFE’s energy and environmental technology sector. The code they have now cracked, is something researchers all over the world are working hard to solve.
The goal is to ensure that silicon can replace graphite and function stable (see more at the bottom) of the negative electrode (anode) in lithium ion batteries (Li-ion). These are the batteries used in electric cars, cell phones, tablets, laptops and most of the time we surround ourselves with technology in everyday life. Success in using silicon in these batteries will thus provide far better capacity. Theoretically, silicon has 10 times as high capacity as graphite, but so far, the problem has been that the silicon bursts up and is destroyed when it is recharged.
The breakthrough of IFE is that they have succeeded in making silicon function stable as anode material. This is a result of several years of targeted research and experimental trials with nanoparticles, including silicon, in IFE’s laboratories at Kjeller.
“Within the new nanoparticles, there is a finely divided mixture of silicon and another material that we would like to call the matrix. This matrix will help silicon to withstand the big volume change it goes through when it is discharged and discharged, “says Asbjørn Ulvestad, researcher and leader of IFE’s research efforts in the SiliconX project and one of the inventors behind the unique technology.
The plan is now, in collaboration with Kjeller Innovation, to investigate which business models may be relevant, while further developing the material ‘in-house’ at Kjeller. In addition, it is working to patent the technology and test it further.
More about the challenge
Theoretically, silicon has a high potential as anode material, with 10 times as high capacity as graphite, which is today’s commercial material. The problem is that the silicon particles expand by up to 400% during charging, and retreat during discharge. This tolerates the particles poorly, and after repeated extensions, the particles break. See illustration in the figure below, with test results from pure silicon batteries in the anode (top curve). IFE now believes that it has succeeded in making silicon function stable as anode material.
With the developed method, you get increased stability in exchange for some of the silicon capacity. However, since this is so huge at first, it ends up with a material with 3-5 times higher capacity than the graphite used in today’s batteries.
Translated from the Norwegian
Learn more: IFE’s battery researchers have broken battery code: Can revolutionize range and lifespan
The Latest on: SiliconX
[google_news title=”” keyword=”SiliconX” num_posts=”10″ blurb_length=”0″ show_thumb=”left”]
via Google News
The Latest on: SiliconX
- Feed has no items.
via Bing News