From the polyurethane that makes our car seats to the paper made from bleached wood pulp, chlorine can be found in a variety of large-scale manufacturing processes. But while chlorine is good at activating the strong bonds of molecules, which allows manufacturers to synthesize the products we use on a daily basis, it can be an insidious chemical, sometimes escaping into the environment as hazardous byproducts such as chloroform and dioxin.
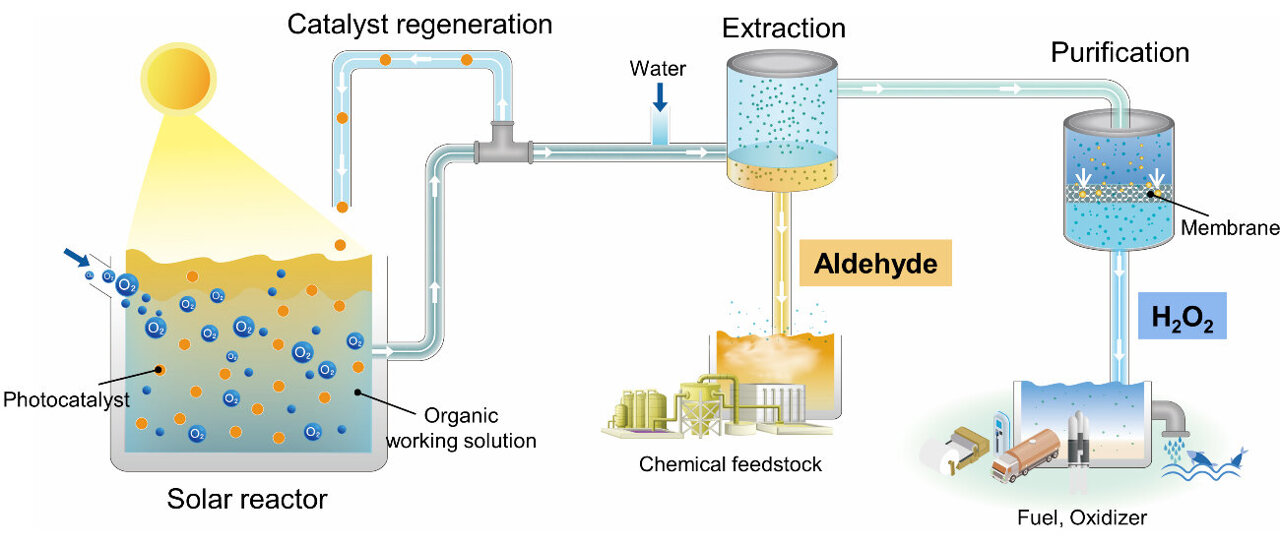
As a result, scientists and companies have been exploring a more environmentally benign alternative to chlorine—hydrogen peroxide, or H2O2. But it is an expensive reactant. Hydrogen peroxide is typically made in big, centralized facilities and requires significant energy for separation, concentration, and transportation. A handful of large-scale facilities around the globe have begun to produce H2O2 using the current process, but at the same facilities as the polyurethane precursors, which results in significant cost and energy savings and reduces environmental impact. Ideally smaller-scale factories would also be able to make hydrogen peroxide on site, but this would require a completely different set of chemistry, direct synthesis of H2O2 from hydrogen and oxygen gas, which has long been poorly understood according to University of Illinois researchers.
New research from David Flaherty, assistant professor of chemical and biomolecular engineering, and graduate student Neil Wilson reveals the mechanism for the direct synthesis of H2O2 on palladium cluster catalysts, and paves the way to design improved catalysts to produce H2O2 to use in place of harmful chlorine, regardless of the scale of the production facility. The research appears as the cover story for the Jan. 20, 2016 issue of the Journal of the American Chemical Society.
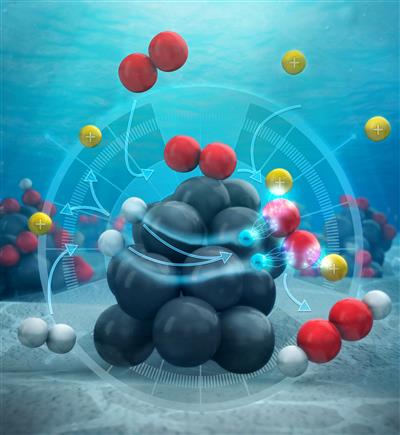
The commonly accepted mechanism for direct synthesis of H2O2 essentially states that hydrogen and oxygen atoms bind adjacent to one another on the catalyst surface and then react, Wilson said. To better understand what was going on, he spent over a year building a reactor, fine-tuning experimental procedures, then collecting and analyzing reaction rate data.
“What people thought was happening is after the hydrogen atoms broke apart and they’re adsorbed onto the palladium surface, that they just reacted with the oxygen on the surface. But that’s not really consistent with what we saw,” said Wilson, a fourth-year graduate student in Flaherty’s lab and first author of the article, “Mechanism for the Direct Synthesis of H2O2 on Pd Clusters: Heterolytic Reaction Pathways at the Liquid–Solid Interface.”
Featured on the journal’s cover is an image that depicts their findings: Instead of reacting together on the surface of the catalyst (the palladium cluster), the hydrogen atoms dissociate into their components—protons and electrons. The protons enter the surrounding solution of water and methanol, while the electrons flow through the palladium itself into oxygen molecules.
“When oxygen comes down onto the surface, it can react with pairs of protons and electrons to form hydrogen peroxide,” Wilson said.
“The reason this is critical,” Flaherty said, “is because it gives us guidance for how to make the next generation of these materials. This is all motivated by trying to make hydrogen peroxide more cheaply so it can be manufactured more easily, so we can use it in place of chlorine. But we didn’t know how to go about making a catalyst that was better than what we have now.”
Read more: Research reveals mechanism for direct synthesis of hydrogen peroxide
The Latest on: Hydrogen peroxide as an alternative to chlorine
[google_news title=”” keyword=”hydrogen peroxide as an alternative to chlorine” num_posts=”10″ blurb_length=”0″ show_thumb=”left”]
via Google News
The Latest on: Hydrogen peroxide as an alternative to chlorine
- Peroxide Codes (April 2024)on April 27, 2024 at 9:27 pm
Peroxide bestowed upon me the honor of becoming an actual Soul Reaper. I got the ultimate Bleach experience in this game by choosing races from the show, mastering abilities, and gaining a high ...
- How to deep clean your mattress safely with hydrogen peroxide — a step by step guideon April 22, 2024 at 2:59 am
Hydrogen peroxide is a safe and effective way to clean your mattress, if used correctly. For the best results, follow our step by step guide.
- Evonik launches certified carbon-neutral hydrogen peroxideon April 17, 2024 at 8:38 am
Evonik Industries AG (Essen, Germany) is now offering carbon-neutral hydrogen peroxide to customers in Europe. This has been enabled through an ...
- How to Use Hydrogen Peroxide for Plants (Hint: Experts Say Sparingly)on April 5, 2024 at 5:01 am
Although articles on hydrogen peroxide for plants abound, it actually has few proven gardening-related uses. As Jeff Gillman, Ph.D., author of The Truth About Garden Remedies and director of the ...
- Hydrogen Peroxide in Ear: How Well Does It Work?on April 4, 2024 at 5:00 pm
Hydrogen peroxide, a liquid used for cleaning and personal care, can help soften earwax and make it easier to remove. Earwax is a natural substance the body produces to protect and keep the ears ...
- 22 Ingenious Ways To Use Hydrogen Peroxideon March 26, 2024 at 12:46 pm
Hydrogen peroxide is a hidden gem when it comes to budget-friendly and versatile household cleaners. Not only is it effective in tackling a wide range of cleaning tasks, but it’s also a safe ...
- 5 Reasons to Use Hydrogen Peroxide for Plants Both Indoors and Outon February 28, 2024 at 3:42 am
Put away the pricey plant food because you can use hydrogen peroxide for plants in a myriad of ways ... by this household basic's properties and benefits. It's an affordable alternative to more ...
- How to Invest in Hydrogen, an Alternative to Fossil Fuel Energyon August 2, 2022 at 5:00 pm
Hydrogen is a promising alternative energy source to fossil fuels but is in direct competition with lithium-based batteries. Hydrogen is touted as an alternative and clean energy source that can ...
- Stop Inhaling Hydrogen Peroxide To Try To Prevent, Treat Covid-19, AAFA Sayson October 2, 2021 at 11:07 am
And don’t inhale hydrogen peroxide to try to prevent or treat Covid-19. These three things should be obvious. Nevertheless, the Asthma and Allergy Foundation of America (AAFA) has had to issue a ...
- Summer Safety: Protecting Your Family from Environmental Health Riskson December 22, 2020 at 2:47 am
As you prepare to let your kids explore the great outdoors this summer, you may have some nagging worries. What chemicals and environmental toxins might lurk in the local pool, lake, or beach?
via Bing News