Developing an efficient way to freeze and store living tissues could transform many aspects of medical care and research
Developing an efficient way to freeze and store living tissues could transform many aspects of medical care and research, but ice crystallization often occurs within cells during such cryopreservation procedures, leading to cell death. In the November 5 issue of the Biophysical Journal, a Cell Press publication, researchers report that they have gained new information about the processes that are responsible for promoting the freezing of cells within tissues. This knowledge may ultimately lead to novel approaches for preventing tissue injury during cryopreservation.
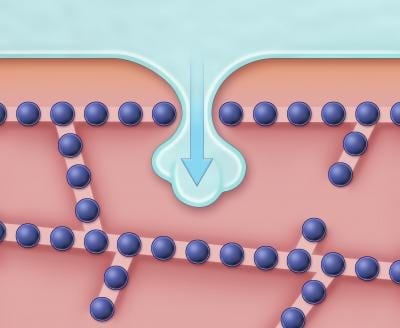
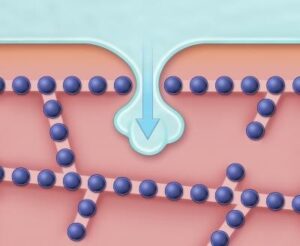
A long-standing obstacle to avoiding tissue damage during freezing is that when cells are joined together within tissues, individual cells are more likely to crystallize than if the cells are kept apart. “In tissues, ice crystals are thought to be able to grow through membrane channels called gap junctions, thus allowing ice to easily propagate from cell to cell,” explains senior author Dr. Jens Karlsson, of the Department of Mechanical Engineering at Villanova University. “But the results of the present study indicate that the mechanism of tissue cryo-injury is much more complex than was previously thought.”
Dr. Karlsson and his team monitored microscopic freezing events inside genetically modified cells and used mathematical models to show that gap junctions do not always provide the major pathway for the spreading of ice crystals between cells. They saw that cell-to-cell propagation of ice also occurred during freezing of tissue samples in which gap junction formation had been suppressed. The authors discovered that intercellular connections—in which neighboring cell membranes are stitched together by rows of rivet-like structures known as tight junctions—also play a significant role.
“By using high-speed video imaging, we found evidence that ice from outside the cells sometimes forms nanoscale branches, which can penetrate the barriers created by tight junction seams,” says Dr. Karlsson. “The resulting invasion of the spaces between cells appears to promote crystallization of the cells adjacent to the breach.”
The unexpected findings may provide a boon for the manufacturing of engineered tissue products that can be used for grafts and organ transplantations. “Using cryopreservation to stop living tissue constructs from spoiling during storage will be the key to enabling economical mass production, quality assurance, and shipping logistics for these life-saving products,” says Dr. Karlsson.
Go deeper with Bing News on:
Tissue cryopreservation
- Buy Rating Affirmed: LeMaitre Vascular’s Growth and Profitability Outlook
Stifel Nicolaus analyst Rick Wise upgraded the rating on Lemaitre Vascular (LMAT – Research Report) to a Buy today, setting a price ...
- Delaying menopause: Groundbreaking women’s health research underway
From hot flashes and mood shifts to more serious symptoms, menopause can be a challenging chapter for many women. Now, a Yale scientist hopes to re-calculate the average age of onset using a technique ...
- I went through fertility treatments for 10 years, and I'd do it all over again
During almost a decade in trying to have my two kids, I endured a total of 17 attempted cycles to conceive. I underwent 38 procedures, had 15 failed cycles of fertility treatment, nine embryo ...
- Asia Pacific Stem Cell Therapy Market Anticipates US$ 3,420.05 Million Value by 2031
The Asia Pacific Stem Cell Therapy Market is poised for substantial growth in the coming years, with a projected compound annual growth rate (CAGR) of 10.29% between 2023 and 2031. This growth is ...
- Experimental Ovarian Tissue Freezing Could Delay Menopause, but Experts Are Weighing the Risks
Extracting, freezing and retransplanting slices of hormone-producing ovarian tissue could postpone menopause, but some experts say it’s not effective enough—or necessary ...
Go deeper with Google Headlines on:
Tissue cryopreservation
[google_news title=”” keyword=”tissue cryopreservation” num_posts=”5″ blurb_length=”0″ show_thumb=”left”]
Go deeper with Bing News on:
Cryopreservation
- Researchers Unveil Automated Embryo Injection Technology with Broad Applications
technology could be used not merely as a tool in genetic experiments but also as an aid in avoiding the extinction of many species through cryopreservation, a methodology whose effectiveness has been ...
- Scientists Are Building a Noah’s Ark for Corals
One effort includes a “Noah’s Ark” for corals—executed by placing rare, threatened species in aquariums in Europe as part of the World Coral Conservatory Project.
- Cell Cryopreservation - Global Market Analysis to 2030 Featuring Profiles of Merck, Thermo Fisher Scientific, Sartorius, PromoCell, and Lonza Group
The "Global Cell Cryopreservation Market Size, Share & Industry Trends Analysis, 2023-2030" report has been added to ResearchAndMarkets.com's offering.The Global Cell Cryopreservation Market is ...
- From a Backup Technology to a Strategy-Outlining Approach
During the past decade, cryopreservation of oocytes and embryos has become one of the most exciting areas in human-assisted reproduction. Improvement of the old techniques and introduction of ...
- These Are the Top 12 Fertility Clinics in the Northeast Region of the US
Accessibility is essential for patients undergoing fertility treatments. Knowing the best clinics in a certain region can help them make informed decisions.
Go deeper with Google Headlines on:
Microplastics in water
[google_news title=”” keyword=”microplastics in water” num_posts=”5″ blurb_length=”0″ show_thumb=”left”]