This vial of clear, sodium-ion electrolyte provides the circulating “blood” that keeps the energy flowing through an experimental battery technology.
(Photo by Andrea Starr | Pacific Northwest National Laboratory)
Cheap and abundant, sodium is a prime promising candidate for new battery technology. But limited performance of sodium-ion batteries has hindered their large-scale applications.
Now, a research team from the Department of Energy’s Pacific Northwest National Laboratory has developed a sodium-ion battery with greatly extended longevity in laboratory tests. An ingenious shift in the ingredients that make up the liquid core of the battery prevents the performance issues that have bedeviled sodium-based batteries. The findings, described in the journal Nature Energy, provide a promising recipe for a battery that may one day power electric vehicles and store energy from the sun.
“Here, we have shown in principle that sodium-ion batteries have the potential to be a long lasting and environmentally friendly battery technology,” said PNNL lead author Jiguang (Jason) Zhang, a pioneer of battery technologies with more than 23 patented inventions in energy storage technology.
The right salt
In batteries, electrolyte is the circulating “blood” that keeps the energy flowing. The electrolyte forms by dissolving salts in solvents, resulting in charged ions that flow between the positive and negative electrodes. Over time, the electrochemical reactions that keep the energy flowing get sluggish, and the battery can no longer recharge. In current sodium-ion battery technologies, this process happens much faster than in similar lithium-ion batteries.
Battery charging
As a battery goes through repeated cycles of charging and discharging, it loses its ability to hold a charge. A new sodium-ion battery technology developed at Pacific Northwest National Laboratory holds its ability to charge for longer than previously described sodium-ion batteries. (Animation by Sara Levine | Pacific Northwest National Laboratory)
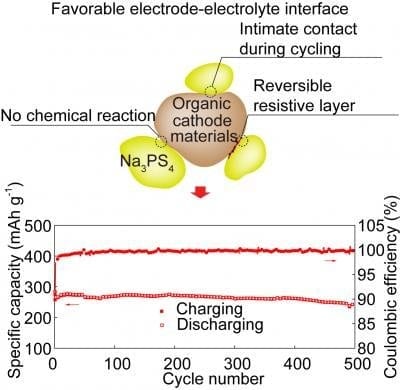
The PNNL team, led by scientists Yan Jin and Phung Le, attacked that problem by switching out the liquid solution and the type of salt flowing through it to create a wholly new electrolyte recipe. In laboratory tests, the new design proved durable, holding 90 percent of its cell capacity after 300 cycles at 4.2 V, which is higher than most sodium-ion batteries previously reported.
The current electrolyte recipe for sodium-ion batteries results in the protective film on the negative end (the anode) dissolving over time. This film is critical because it allows sodium ions to pass through while preserving battery life. The PNNL-designed technology works by stabilizing this protective film. The new electrolyte also generates an ultra-thin protective layer on the positive pole (the cathode) that contributes to additional stability of the entire unit.
Non-flammable technology
The new PNNL-developed sodium-ion technology uses a naturally fire-extinguishing solution that is also impervious to temperature changes and can operate at high voltages. One key to this feature is the ultra-thin protective layer that forms on the anode. This ultra-thin layer remains stable once formed, providing the long cycle life reported in the research article.
Cheap and abundant, sodium is a prime promising candidate for new battery technology. But limited performance of sodium-ion batteries has hindered their large-scale applications.
Now, a research team from the Department of Energy’s Pacific Northwest National Laboratory has developed a sodium-ion battery with greatly extended longevity in laboratory tests. An ingenious shift in the ingredients that make up the liquid core of the battery prevents the performance issues that have bedeviled sodium-based batteries. The findings, described in the journal Nature Energy, provide a promising recipe for a battery that may one day power electric vehicles and store energy from the sun.
“Here, we have shown in principle that sodium-ion batteries have the potential to be a long lasting and environmentally friendly battery technology,” said PNNL lead author Jiguang (Jason) Zhang, a pioneer of battery technologies with more than 23 patented inventions in energy storage technology.
The right salt
In batteries, electrolyte is the circulating “blood” that keeps the energy flowing. The electrolyte forms by dissolving salts in solvents, resulting in charged ions that flow between the positive and negative electrodes. Over time, the electrochemical reactions that keep the energy flowing get sluggish, and the battery can no longer recharge. In current sodium-ion battery technologies, this process happens much faster than in similar lithium-ion batteries.
Battery charging
As a battery goes through repeated cycles of charging and discharging, it loses its ability to hold a charge. A new sodium-ion battery technology developed at Pacific Northwest National Laboratory holds its ability to charge for longer than previously described sodium-ion batteries.
The PNNL team, led by scientists Yan Jin and Phung Le, attacked that problem by switching out the liquid solution and the type of salt flowing through it to create a wholly new electrolyte recipe. In laboratory tests, the new design proved durable, holding 90 percent of its cell capacity after 300 cycles at 4.2 V, which is higher than most sodium-ion batteries previously reported.
The current electrolyte recipe for sodium-ion batteries results in the protective film on the negative end (the anode) dissolving over time. This film is critical because it allows sodium ions to pass through while preserving battery life. The PNNL-designed technology works by stabilizing this protective film. The new electrolyte also generates an ultra-thin protective layer on the positive pole (the cathode) that contributes to additional stability of the entire unit.
Non-flammable technology
The new PNNL-developed sodium-ion technology uses a naturally fire-extinguishing solution that is also impervious to temperature changes and can operate at high voltages. One key to this feature is the ultra-thin protective layer that forms on the anode. This ultra-thin layer remains stable once formed, providing the long cycle life reported in the research article.
Original Article: Longer Lasting Sodium-Ion Batteries on the Horizon
More from: Pacific Northwest National Laboratory
The Latest Updates from Bing News & Google News
Go deeper with Bing News on:
Sodium-ion batteries
- New Progress in Sodium-ion Battery Electrolyte Research
On May 7th, the Dalian Institute of Chemical Physics of the Chinese Academy of Sciences announced new progress in the research of sodium-ion battery electrolytes. The sodium vanadium ...
- Why the use of sodium-ion batteries is set to expand
Why the use of sodium-ion batteries is set to expand The idea of sodium-ion battery production is starting to take off, with the development of models for both industry and electric mobility. Beyond ...
- 🔋 This battery recharges in seconds: the revolution for smartphones and electric vehicles?
A significant advancement in battery technology could soon revolutionize smartphone and electric vehicle technologies. Researchers have developed a new sodium-based battery capable of recharging ...
- CATL Explores Tech Licensing With Automakers As It Focuses On Sodium-Ion Batteries
Chinese power battery giant Contemporary Amperex Technology Co Ltd (CATL) has reportedly initiated discussions with approximately twelve car manufacturers regarding ...
- German initiative to develop scalable sodium-ion batteries
A collaborative project led by German battery supplier Varta aims to develop industrial-scale sodium-ion battery technology. The final product of the three-year, €7.5 million ($8.08 million) project ...
Go deeper with Google Headlines on:
Sodium-ion batteries
[google_news title=”” keyword=”sodium-ion batteries” num_posts=”5″ blurb_length=”0″ show_thumb=”left”]
Go deeper with Bing News on:
Sodium-based batteries
- New Progress in Sodium-ion Battery Electrolyte Research
On May 7th, the Dalian Institute of Chemical Physics of the Chinese Academy of Sciences announced new progress in the research of sodium-ion battery electrolytes. The sodium vanadium ...
- Sodium battery startup wins People’s Choice Award at Industry Growth Forum
Adena Power is developing an energy storage solution using sodium batteries and domestically-sourced raw materials.
- 🔋 This battery recharges in seconds: the revolution for smartphones and electric vehicles?
A significant advancement in battery technology could soon revolutionize smartphone and electric vehicle technologies. Researchers have developed a new sodium-based battery capable of recharging ...
- CATL Explores Tech Licensing With Automakers As It Focuses On Sodium-Ion Batteries
Chinese power battery giant Contemporary Amperex Technology Co Ltd (CATL) has reportedly initiated discussions with approximately twelve car manufacturers regarding ...
- Sodium-Ion Battery Advances Pave the Way for Wide Adoption by the Automotive Industry
Sodium-ion batteries promise to be cheap and safe, but they need further development to replace lithium-ion batteries that power electric vehicles ...
Go deeper with Google Headlines on:
Sodium-based batteries
[google_news title=”” keyword=”sodium-based batteries” num_posts=”5″ blurb_length=”0″ show_thumb=”left”]