Building ‘Smart’ Cell-Based Therapies
A Northwestern synthetic biology team has created a new technology for modifying human cells to create programmable therapeutics that could travel the body and selectively target cancer and other sites of disease.
Engineering cell-based, biological devices that monitor and modify human physiology is a promising frontier in clinical synthetic biology. However, no existing technology enabled bioengineers to build such devices that sense a patient’s physiological state and respond in a customized fashion.
“The project addressed a key gap in the synthetic biology toolbox,” says Joshua Leonard, assistant professor of chemical and biological engineering in Northwestern’s McCormick School of Engineering and Applied Science. “There was no way to engineer cells in a manner that allowed them to sense key pieces of information about their environment, which could indicate whether the engineered cell is in healthy tissue or sitting next to a tumor.”
Funded by the National Academies Keck Futures Initiative and the Defense Advanced Research Projects Agency, the research is available to read online in the journal ACS Synthetic Biology.
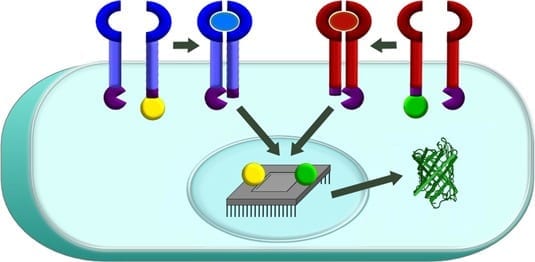
Leonard’s team worked for nearly four years to close this gap. The end result is a protein biosensor that sits on the surface of a cell and can be programmed to sense specific external factors. For example, the engineered cell could detect big, soluble protein molecules that indicate that it’s next to a tumor. When the biosensor detects such a factor, it sends a signal into the engineered cell’s nucleus to activate a gene expression program, such as the production of tumor-killing proteins or chemicals. Since this toxic program would be activated only near tumor cells, such an approach could minimize side effects as well as improve therapeutic benefits.
Called a Modular Extracellular Sensor Architecture (MESA), the biosensor platform is completely self-contained so that several different biosensors can be present in a single cell without interfering with one another, allowing bioengineers to build increasingly sophisticated functional programs. The platform is also highly modular, enabling the biosensors to be customized to recognize factors of relevance to various patients’ needs.
“By linking the output of these biosensors to genetic programs, one can build in a certain logical command, such as ‘turn the output gene on when you sense this factor but not that factor,’” Leonard explains. “In that way, you could program a cell-based therapy to specify which cells it should kill.”
Leonard says doctors could potentially collect immune cells from a patient’s body, engineer the cells using MESA, and put them back into the patient. From there, the cells would do the work of detecting cancer or the disease they are designed to identify.
The Latest on: Smart Cell-Based Therapies
[google_news title=”” keyword=”Smart Cell-Based Therapies” num_posts=”10″ blurb_length=”0″ show_thumb=”left”]
via Google News
The Latest on: Smart Cell-Based Therapies
- Woman describes toll of continued cancer treatments on her mental healthon April 30, 2024 at 1:02 pm
Mental health awareness month kicks off this week. For one breast cancer survivor, that means taking care of the body – and the brain.
- Global Scaffold Technology Market is set to expand at a CAGR of 11.3% until 2034 | Future Market Insights, Inc.on April 30, 2024 at 3:45 am
The scaffold technology industry is expected to be valued at US$ 1,490.7 million by 2024. The market valuation is estimated to be US$ 4,348.6 million by 2034, projected at a CAGR of 11.3%.
- Bristol Myers Squibb enlists Cellares amid industry manufacturing “land grab”on April 29, 2024 at 7:54 am
With equipment that promises to produce 10 times more therapies, the deal with Cellares is hoped to make BSM's CAR-T ambitions more scalable and affordable ...
- Bristol Myers builds on "smart factory" bet with new agreementon April 26, 2024 at 7:52 am
Bristol Myers Squibb (NYSE: BMY) has agreed terms for a new CAR-T cell therapy supply agreement worth up to $380 million in upfront and milestone payments. The New Jersey, USA-based cancer company ...
- Overcoming toxicities: are IL-2 smart cytokines the next big thing?on April 24, 2024 at 6:11 am
A more advanced form of IL-2 cytokine therapy called IL-2 smart cytokines could overcome side effects, and shake up cancer care.
- Cutting-edge RM6 Million Laboratory Opens at UMMC to Advance Cancer Treatmenton April 23, 2024 at 11:27 pm
Cancer patients can approach UMMC for enquiries on Auxi Therapeutics GMP CAR-T & CGT Laboratory. Blood cancer patients may contact Consultant Clinical Haematologist Prof Dr Bee Ping Chong, and ...
- Cell Therapy Startup Energized via Smarter Operationson April 23, 2024 at 5:00 pm
A British-based cell therapy company is using smart operations to streamline its viral vector processing. According to Paul S. Carter, senior director, manufacturing science and technology (MSAT ...
- Cell Therapy Startup Energized via Smarter Operationson April 23, 2024 at 5:00 pm
A British-based cell therapy company is using smart operations to streamline its viral vector processing. According to Paul S. Carter, senior director, manufacturing science and technology (MSAT), at ...
- Bristol Myers taps startup to boost cell therapy productionon April 22, 2024 at 4:30 am
A partnership with Cellares, worth up to $380 million, is meant to help Bristol Myers speed and scale manufacture of CAR-T treatments for cancer.
- What is TMS Therapy for Anxiety? A Complete Guide by The Best TMS Center Near Meon April 19, 2024 at 6:12 am
Anxiety refers to the foresight of a future concern and is more associated with muscle tension and avoidance behavior.
via Bing News