Rice lab develops dual-surface graphene electrode to split water into hydrogen and oxygen
Rice University chemists have produced a catalyst based on laser-induced graphene that splits water into hydrogen on one side and oxygen on the other side. They said the inexpensive material may be a practical component in generating the hydrogen for use in future fuel cells.
The easily fabricated material developed by the Rice lab of chemist James Tour offers a robust and efficient way to store chemical energy. Tests showed the thin catalyst producing large bubbles of oxygen and hydrogen on either side simultaneously.
The process is the subject of a paper in the American Chemical Society’s Applied Materials and Interfaces.
“Hydrogen is currently made by converting natural gas to a mixture of carbon dioxide and hydrogen gas,” Tour said. “So for every two hydrogen molecules, a molecule of carbon dioxide is formed, making this traditional process a greenhouse-gas emitter.
“But if one splits water into hydrogen and oxygen, using a catalytic system and electricity generated from wind or solar energy, then the hydrogen afforded is entirely renewable,” he said. “Once used in a fuel cell, it reverts back to water with no other emissions. And fuel cells are often twice as efficient as internal combustion engines, further saving energy.”
The catalyst is another use for versatile laser-induced graphene (LIG), which Rice introduced in 2014. LIG is produced by treating the surface of a sheet of polyimide, an inexpensive plastic, with a laser. Rather than a flat sheet of hexagonal carbon atoms, LIG is a foam of graphene sheets with one edge attached to the underlying surface and chemically active edges exposed to the air.

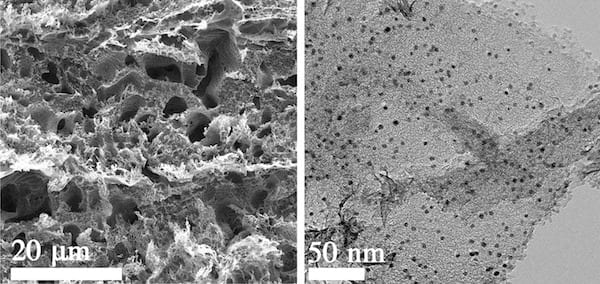
A two-sided electrocatalyst developed at Rice University splits water into hydrogen on one side and oxygen on the other. On the oxygen side, seen in electron microscope images, nickel and iron are deposited onto laser-induced graphene. Click on the image for a larger version. Courtesy of the Tour Group
LIG itself is inert, so turning it into a water splitter involves a few more steps. First, the lab impregnated the side of the plastic destined to pull hydrogen from water with platinum particles; then the lab used a laser to heat the surface and make LIG. The Rice material uses only a quarter of the platinum found in commercial catalysts, said Jibo Zhang, a Rice graduate student and lead author of the paper.
The other side, for oxygen evolution, was first turned into LIG and then enhanced with nickel and iron through electrochemical deposition. Both sides showed low onset potentials (the voltage needed to start a reaction) and strong performance over 1,000 cycles.
The lab came up with another variation: making the polyimide into an LIG catalyst with cobalt and phosphorus that could replace either the platinum or nickel-iron sides to produce hydrogen or oxygen. While the low-cost material benefits by eliminating expensive noble metals, it sacrifices some efficiency in hydrogen generation, Tour said.
When configured with cobalt-phosphorus for hydrogen evolution and nickel-iron for oxygen, the catalyst delivered a current density of 10 milliamps per square centimeter at 1.66 volts. It could be increased to 400 milliamps per square centimeter at 1.9 volts without degrading the material. The current density governs the rate of the chemical reaction.
Tour said enhanced LIG offers water-splitting performance that’s comparable and often better than many current systems, with an advantage in its inherent separator between oxygen and hydrogen products. He noted it may find great value as a way to chemically store energy from remote solar or wind power plants that would otherwise be lost in transmission.
The material might also serve as the basis for efficient electrocatalysis platforms for carbon dioxide or oxygen reduction, he said.
Learn more: 2 sides to this energy story
The Latest on: Water-splitting
[google_news title=”” keyword=”water-splitting” num_posts=”10″ blurb_length=”0″ show_thumb=”left”]- Feds say he masterminded an epic California water heist. Some farmers say he’s their Robin Hoodon April 28, 2024 at 3:00 am
Stolen water is an indelible part of California lore. But the federal case against Dennis Falaschi, longtime head of the Panoche Water District, alleges one of the most audacious water grabs in modern ...
- NIKKI SIXX On MICK MARS Splitting With MÖTLEY CRÜE: "We Really Had To Sit Down And Go, 'Well, What Do We Do? Do We Fold?'"on April 27, 2024 at 8:26 am
The story of Mick Mars and Mötley Crüe splitting goes back to October 2022, when Mars retired as a touring member of the band. John 5 was announced as his replacement the next day, and was later named ...
- 84-year-old man found dead in water at Bronx golf course: Policeon April 26, 2024 at 2:57 pm
An 84-year-old man was found dead in a body of water at a golf course in the Bronx, according to police. The body was discovered after a report of a drowning at the Pelham Bay and Split Rock Golf ...
- Lawmakers split on whether amendment including Water Commission needs is relevanton April 25, 2024 at 11:45 am
Disagreement between the two chambers centers on concerns raised by the state attorney general’s office that the amendment to include the Water Commission is not relevant to the original bill.
- Gov. Whitmer Announces $290 Million Boost for Michigan's Water Infrastructure, Supporting Over 4,000 Jobson April 23, 2024 at 12:50 pm
Michigan receives a $290 million budget boost for water infrastructure, promising cleaner water and 4,350 jobs.
- Commentary: Here’s what FWC missed in Split Oak planon April 23, 2024 at 10:55 am
Florida Fish and Wildlife Conservation Commission has a plan for mitigating damage to Split Oak Forest if a toll road is built there, but the plan has no net positive conservation benefit.
- The Sinking Arizona Town Where Water and Politics Collideon April 23, 2024 at 7:20 am
Democrats see an opening to win back rural Trump voters fed up with their groundwater being pumped by huge farms.
- Gov. Gretchen Whitmer announces $290 million to replace lead pipes, upgrade water systems in Michiganon April 22, 2024 at 11:38 am
(CBS DETROIT) - Gov. Gretchen Whitmer and the Department of Environment, Great Lakes, and Energy (EGLE) announced an expansion that will help communities replace lead pipes, upgrade water systems and ...
- ASUS Launches ROG RYUJIN III WB: A New Split Water-Cooling Solution with LCD Displayon April 21, 2024 at 11:43 pm
ASUS introduced its first split water-cooling product, the ROG RYUJIN III WB, on its official overseas website. This product, first revealed at CES 2024, features a split cold head known as the ...
- Haitians scramble to seek food, water and safety as gang violence chokes the capitalon April 21, 2024 at 11:38 am
Life in Port-au-Prince has become a game of survival, pushing Haitians to new limits as they scramble to stay safe and alive while gangs overwhelm the police and the government remains largely absent ...
via Google News and Bing News