Researchers have worked out how to reprogram cells from human skin into functioning nerve cells.
By transforming cells from human skin into working nerve cells, researchers may have come up with a model for nervous-system diseases and perhaps even regenerative therapies based on cell transplants.
The achievement, reported online today in Nature, is the latest in a fast-moving field called transdifferentiation, in which cells are forced to adopt new identities. In the past year, researchers have converted connective tissue cells found in skin into heart cells, blood cells and liver cells.
Transdifferentiation is an alternative to the cellular reprogramming that involves converting a mature cell into a pluripotent stem cell — one capable of becoming many types of cell — then coaxing the pluripotent cell into becoming a particular type of cell, such as neurons. Marius Wernig, a stem-cell researcher at Stanford University in California, and the leader of the study, says that skipping the pluripotency step could avoid some of the problems of making tissues from these induced pluripotent stem cells (iPSCs). The pluripotency technique can also take months to complete.
Wernig’s team sparked the imaginations of cellular reprogrammers last year, when it transformed cells taken from the tip of a mouse’s tail into working nerve cells. That feat of cellular alchemy took just three foreign genes-delivered into tail cells with a virus-and less than two weeks. “We thought that as it worked so great for the mouse, it should be no problem to work it out in humans,” Wernig says. “That turned out to be wrong.”
Not quite right
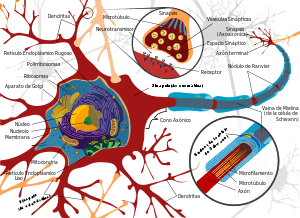
Those three genes also made human cells that looked like nerve cells but that did not fire the electric pulses characteristic of neurons. However, the addition of a fourth virus-delivered gene, found through trial and error, pushed fibroblast cells — connective tissue cells found throughout the body and involved in wound healing — collected from aborted fetuses and the foreskin of newborns to become bona fide neurons. After a couple of weeks in culture, many of the neurons responded to electric jolts by pumping ions across their membranes. A few weeks later still, these neurons started to form connections, or synapses, with the mouse neurons they were grown alongside.
There are still kinks to work out, Wernig admits. Only 2-4% of the fibroblasts became neurons — lower than the roughly 8% efficiency his team achieved with the mouse tail cells. And most of the resulting neurons communicated using a chemical called glutamate, limiting their use for understanding or treating diseases such as Parkinson’s, which is characterized by problems in neurons that communicate with this chemical.
Wernig says that his team expects their efficiency to improve and is trying to make neurons that communicate using other chemicals.