Study uncovers genes that control process of whole-body regeneration
When it comes to regeneration, some animals are capable of amazing feats. If you cut off a salamander’s leg, it will grow back. When threatened, some geckos drop their tails to distract their predator, only to regrow them later.
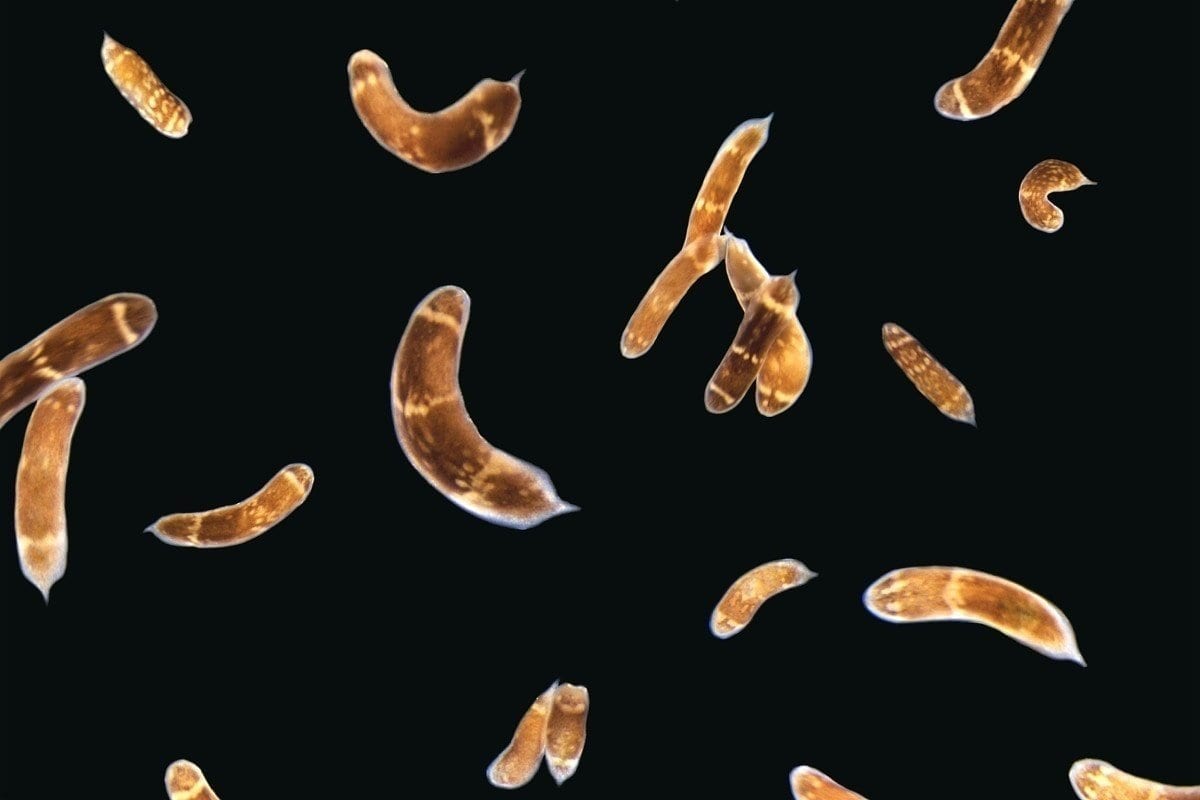
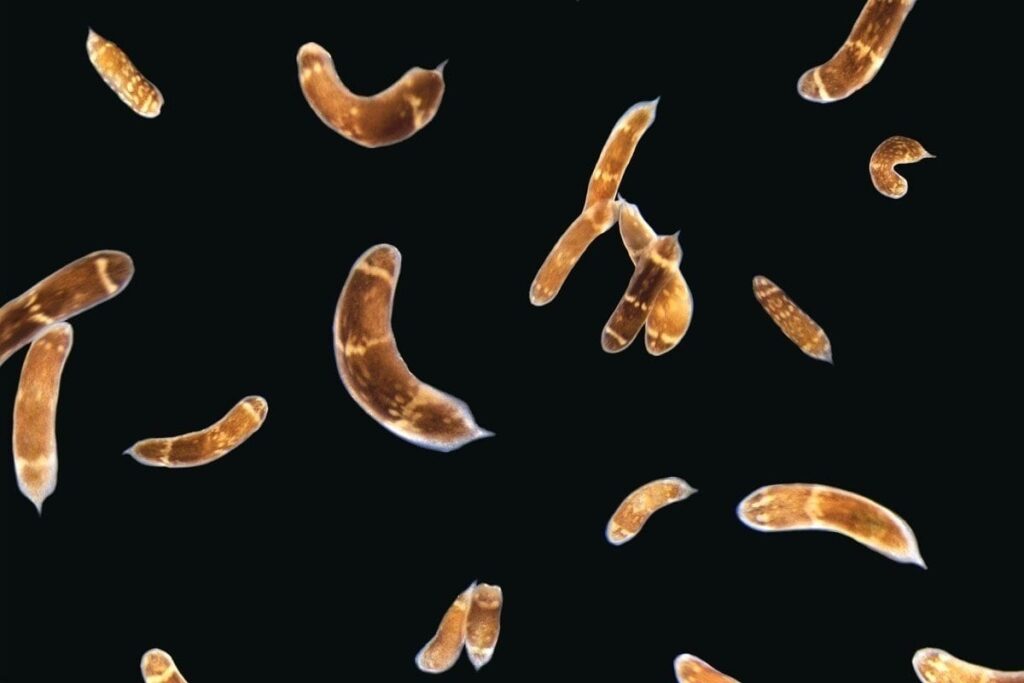
Other animals take the process even further. Planarian worms, jellyfish, and sea anemones can actually regenerate their bodies after being cut in half.
Led by Assistant Professor of Organismic and Evolutionary Biology Mansi Srivastava, a team of researchers is shedding new light on how animals pull off the feat, along the way uncovering a number of DNA switches that appear to control genes for whole-body regeneration. The study is described in a March 15 paper in Science.
Using three-banded panther worms to test the process, Srivastava and Andrew Gehrke, a postdoctoral fellow working in her lab, found that a section of noncoding DNA controls the activation of a “master control gene” called early growth response, or EGR. Once active, EGR controls a number of other processes by switching other genes on or off.
“What we found is that this one master gene comes on [and activates] genes that are turning on during regeneration,” Gehrke said. “Basically, what’s going on is the noncoding regions are telling the coding regions to turn on or off, so a good way to think of it is as though they are switches.”
For that process to work, Gehrke said, the DNA in the worms’ cells, which normally is tightly folded and compacted, has to change, making new areas available for activation.
“A lot of those very tightly packed portions of the genome actually physically become more open,” he said, “because there are regulatory switches in there that have to turn genes on or off. So one of the big findings in this paper is that the genome is very dynamic and really changes during regeneration as different parts are opening and closing.”
Before Gehrke and Srivastava could understand the dynamic nature of the worm’s genome, they had to assemble its sequence — no simple feat in itself.
“That’s a big part of this paper,” Srivastava said. “We’re releasing the genome of this species, which is important because it’s the first from this phylum. Until now there had been no full genome sequence available.”
It’s also noteworthy, she added, because the three-banded panther worm represents a new model system for studying regeneration.
“Previous work on other species helped us learn many things about regeneration,” she said. “But there are some reasons to work with these new worms.” For one thing, they’re in an important phylogenetic position. “So the way they’re related to other animals … allows us to make statements about evolution.” The other reason, she said, is, “They’re really great lab rats. I collected them in the field in Bermuda a number of years ago during my postdoc, and since we’ve brought them into the lab they’re amenable to a lot more tools than some other systems.”
While those tools can demonstrate the dynamic nature of the genome during regeneration — Gehrke was able to identify as many as 18,000 regions that change — what’s important, Srivastava said, is how much meaning he was able to derive from studying them. She said the results show that EGR acts like a power switch for regeneration — once it is turned on, other processes can take place, but without it, nothing happens.
“We were able to decrease the activity of this gene and we found that if you don’t have EGR, nothing happens,” Srivastava said. “The animals just can’t regenerate. All those downstream genes won’t turn on, so the other switches don’t work, and the whole house goes dark, basically.”
While the study reveals new information about how the process works in worms, it also may help explain why it doesn’t work in humans.
“It turns out that EGR, the master gene, and the other genes that are being turned on and off downstream are present in other species, including humans,” Gehrke said.
“The reason we called this gene in the worms EGR is because when you look at its sequence, it’s similar to a gene that’s already been studied in humans and other animals,” Srivastava said. “If you have human cells in a dish and stress them, whether it’s mechanically or you put toxins on them, they’ll express EGR right away.”
The question is, Srivastava said, “If humans can turn on EGR, and not only turn it on, but do it when our cells are injured, why can’t we regenerate? The answer may be that if EGR is the power switch, we think the wiring is different. What EGR is talking to in human cells may be different than what it is talking to in the three-banded panther worm, and what Andrew has done with this study is come up with a way to get at this wiring. So we want to figure out what those connections are, and then apply that to other animals, including vertebrates that can only do more limited regeneration.”
Going forward, Srivastava and Gehrke said they hope to investigate whether the genetic switches activated during regeneration are the same as those used during development, and to continue working to better understand the dynamic nature of the genome.
Learn more: The genetics of regeneration
The Latest on: Whole-body regeneration
[google_news title=”” keyword=”whole-body regeneration” num_posts=”10″ blurb_length=”0″ show_thumb=”left”]
via Google News
The Latest on: Whole-body regeneration
- New Forest District Council leader Cllr Jill Cleary pressed on benefits of Solent Freeport to residents and businesseson April 27, 2024 at 7:00 pm
Speaking at a meeting of New Forest District Council, Cllr Sean Cullen asked leader Cllr Jill Cleary which type of businesses were being encouraged to be a part of the freeport.
- 'The Departed' Director Martin Scorsese Recommends These Compelling Movieson April 27, 2024 at 5:00 pm
While he’s produced an immaculate body of work since his career began in the 1960s, Scorsese also has a reputation for his dedication to the film medium as a whole, possessing ... the cycles of birth ...
- Salt Water Trick For Sleep And Metabolic Regeneration: Does Renew Salt Water Trick Improve Sleep Quality?on April 25, 2024 at 7:33 pm
This solution cleanses the whole body by filtering out toxins and contaminants accumulated ... Well, the recently launched health supplement called Renew Metabolic Regeneration Formula claims to be ...
- New experimental evidence unlocks a puzzle in vascular tissue engineeringon April 25, 2024 at 9:25 am
Angiogenesis is a process of forming hierarchical vascular networks in living tissues. Its complexity makes the controlled generation of blood vessels in laboratory conditions a highly challenging ...
- I just tried two minutes of cryotherapy for the first time — here's what happened to my bodyon April 25, 2024 at 2:04 am
As little as two minutes spent in a cryotherapy chamber could trigger endorphin release, boost mood and reduce pain. You may notice immediate benefits after just one session, such as a boost in energy ...
- Novaa Lab Reviews – Does It Work For Pain Relief or Fake Red Light Therapy Product?on April 25, 2024 at 1:31 am
NovaaLab stands as a beacon of hope for those seeking natural and effective solutions for pain relief and accelerated recovery. Founded by a visionary biohacker, NovaaLab has pioneered the use of red ...
- The murky, unregulated world of anti-ageing stem cell therapyon April 24, 2024 at 10:50 am
Stem cells are the new focal point of the rich and famous with Hollywood A-listers reportedly spending tens of thousands of pounds each year on expensive therapies offered by private longevity clinics ...
- The 23 Best Tattoo Lotions in 2024on April 22, 2024 at 1:30 pm
Branded content. Us Weekly has affiliate partnerships so we may receive compensation for some links to products and services. Tattoo care is the most important part of the whole process to make sure ...
- FedUp Foods: A Cup Half Full Of Blue Ocean Opportunity & Impacton April 22, 2024 at 10:34 am
“So,” Gray says of the now codified purpose, it is “to craft delicious, high quality, functional beverages ,” which in 2021 became the fastest-growing market in the entire food sector. Simultaneously, ...
- Immunotherapy Regenerative Medicine Clinic on Using Stem Cells to Activate the Body's Natural Healing Poweron April 18, 2024 at 10:02 am
Stem cell therapy, a cornerstone of regenerative medicine, has shown immense potential in treating autoimmune diseases, aging conditions, and ...
via Bing News