Gives us greater flexibility in creating effective drugs
A team of scientists anchored at Yale University has demonstrated a new, highly versatile approach for quickly assembling drug-like compounds, establishing a broad new route to drug discovery and medical treatment. They report their results in the journal Science on Feb 8.
Drug molecules interact with their targets, such as proteins or enzymes, by attaching to them in a way that neutralizes the target’s undesirable effects in the body. This is sometimes called the “lock-and-key” method. The new approach offers scientists far greater control over the three-dimensional structure of a key class of molecular compounds, making it easier to fashion drug molecules that fit their targets in the right way.
“Now we’ve got a lot more control over the shape and orientation of this class of drug compounds, and this essentially gives us greater flexibility in creating effective drugs,” said Jonathan Ellman, the Yale chemist who led the experiment.
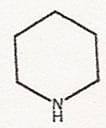
The research reported in Science revolves around piperidines, a class of organic compounds widely used in pharmaceuticals, including the familiar drugs quinine, morphine, oxycodone, Plavix, Cialis, and Aricept. Piperidines are core structures, or scaffolds, upon which molecular fragments — parts of the drug molecule — can be displayed for binding to a drug’s targets. The scientists have shown a way to generate different piperidine derivatives by varying acid strength.
“Our research allows us to make new piperidines easily,” Ellman said. “The approach has biomedical relevance because the scaffold upon which the fragments are displayed is present in many of the most important drugs.”
via Yale University & EurekaAlert
The Latest Streaming News: Piperidines updated minute-by-minute
Bookmark this page and come back often
Latest NEWS
Latest VIDEO







