Cellceutix believes it has found the Holy Grail in cancer research
. . . a compound that activates a tumor suppressor protein known as p53, long regarded as possibly holding the key to future cancer therapies.
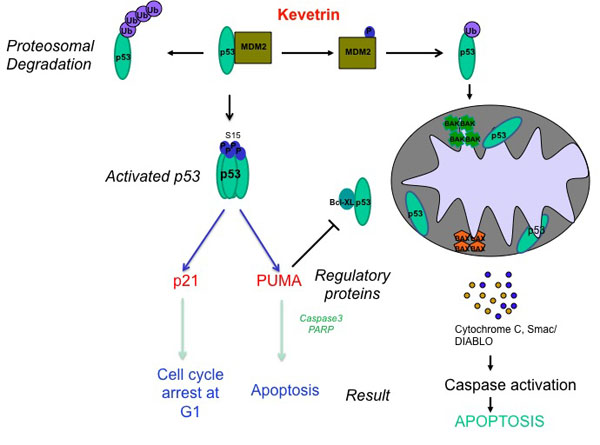
“Our Kevetrin drug reactivates p53 to its normal function of dictating whether to fix the cell or kill the cell,” CEO Leo Ehrlich says in an exclusive interview with BioTuesdays.com. “This has been one of the Holy Grails in cancer research.”
p53 often has been described as the “guardian angel of the human genome” because of its crucial role in regulating the cell cycle and controlling cell mutations. Pharma has spent hundreds of millions of dollars researching ways to reactivate p53, without success. A major stumbling block has been damage to DNA.
Basing his observation on studies of human solid tumors in animals, Mr. Ehrlich says that Kevetrin does not damage DNA and affects both wild type and p53. Wild type refers to the normally functioning gene product, whereas mutant refers to the mutated form of the gene product where normal function is abrogated.
By activating wild type p53, tumor regression is expected because wild type p53 acts a functional transcriptional factor for tumor suppression. Wild type p53 can also function as tumor suppressor by stabilizing the p53 protein levels. Thus wild type p53 acts in both a transcriptional dependent and independent manner for tumor suppression.
Many tumors have mutations in p53; the majority of mutant p53 are defective in suppressing tumor growth because of the defect in transcription. Therefore, drugs which activate p53 in a transcriptional dependent manner are ineffective in mutant p53 tumors. However, Kevetrin stabilizes the mutant p53, allowing mutant p53 to induce tumor suppression in non-transcriptional dependent manner.
Kevetrin addresses one of Pharma’s largest markets: drug-resistant cancers. “When we tested Kevetrin against drug resistant cancer lines on animals using human xenograft tumors, we were able to shrink the tumor,” he points out. He further states, “We are not dealing with just another cancer drug. We have indications that it is effective against drug-resistant cancers, which is a multi-billion-dollar market. Most new cancer drugs are variations of existing cancer drugs, but we have a completely new and novel compound.”
In 2009, the American Association of Cancer Research recognized Kevetrin as one of the “Frontiers” in cancer research because of its promising potency and low toxicity.
Extensive preclinical research on Kevetrin has resulted in a compilation of data showing a wide therapeutic index through the re-activation of p53 and no development of drug resistance. Research has shown Kevetrin to outperform current chemotherapies in testing against multiple cancer lines, including breast, lung, head and neck, colon, prostate and pancreatic cancers and leukemia, Mr. Ehrlich notes.
Asked why the company’s preclinical data makes him optimistic that the drug will work in humans, Mr. Ehrlich says a common trait of a successful drug is that it works across the board against many different cancer types. He says, “What we’ve consistently found with Kevetrin is that in every human cancer line that we’ve tested in animals, the drug has always been effective. That gives us a very high level of confidence that this will make it through.”
Cellceutix is also counting on its chief scientific officer, Dr. Krishna Menon, who developed Kevetrin. From 1995 to 2001, he was Group Leader, Cancer In Vivo Research and Clinical Development, for Eli Lilly, where he played a key role in the selection and preclinical development of Gemzar and Alimta, two blockbuster cancer drugs. In 1999, Lilly honored Dr. Menon with the President’s Recognition Award, the most prestigious award at Eli Lilly.
Earlier this month, Cellceutix filed an IND with the FDA for a Phase 1 clinical trial to test Kevetrin against a variety of different cancer types in patients with advanced-stage cancers. Primary endpoints for the study will be safety, tolerable dosing levels and establishing the dose for a future Phase 2 clinical trial. The IND also includes a provision to study the efficacy of Kevetrin in the patients.
“We’ve already had a lot of interest from Big Pharma,” Mr. Ehrlich commented. He adds, “Even before we filed the IND, some of largest biotechs in the world contacted us, wanting to be apprised of what’s happening during the clinical trial. As the Phase 1 progresses, we expect the interest to continue to escalate.”
Read more . . .
Bookmark this page for “Cancer therapy” and check back regularly as these articles update on a very frequent basis. The view is set to “news”. Try clicking on “video” and “2” for more articles.