CREDIT
Liming Dai
Cheaper, more durable catalyst for fuel cell used in cars, at data centers
For nearly half a century, scientists have been trying to replace precious metal catalysts in fuel cells. Now, for the first time, researchers at Case Western Reserve University have shown that an inexpensive metal-free catalyst performs as well as costly metal catalysts at speeding the oxygen reduction reaction in an acidic fuel cell.
The carbon-based catalyst also corrodes less than metal-based materials and has proved more durable.
The findings are major steps toward making low-cost catalysts commercially available, which could, in turn, reduce the cost to generate clean energy from PEM fuel cells–the most common cell being tested and used in cars and stationary power plants. The study will be published online in the journal Science Advances at 2 p.m. U.S. Eastern Time Friday, Feb. 27.
“This definitely should move the field forward,” said Liming Dai, the Kent Hale Smith Professor of macromolecular science and engineering at Case Western Reserve and senior author of the research. “It’s a major breakthrough for commercialization.”
Dai worked with lead investigator Jianglan Shui, who was a CWRU postdoctoral researcher and is now a materials science and engineering professor at Beihang University, Beijing; PhD student Min Wang, who did some of the testing; and postdoctoral researcher Fen Du, who made the materials. The effort builds on the Dai lab’s earlier work developing carbon-based catalysts that significantly outperformed platinum in an alkaline fuel cell.
The group pursued a non-metal catalyst to perform in acid because the standard bearer among fuel cells, the PEM cell, uses an acidic electrolyte. PEM stands for both proton exchange membrane and polymer electrolyte membrane, which are interchangeable names for this type of cell.
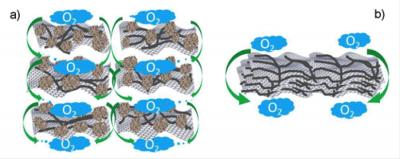
The key to the new catalyst is its rationally-designed porous structure, Dai said. The researchers mixed sheets of nitrogen-doped graphene, a single-atom thick, with carbon nanotubes and carbon black particles in a solution, then freeze-dried them into composite sheets and hardened them.
Read more: CWRU researchers bring clean energy a step closer
The Latest on: PEM fuel cells
[google_news title=”” keyword=”PEM fuel cells” num_posts=”10″ blurb_length=”0″ show_thumb=”left”]
via Google News
The Latest on: PEM fuel cells
- Platinum-based green hydrogen emerging as Winter Olympics decarbonisation pillaron May 3, 2024 at 7:00 am
Electrolysis, transport, storage, marine fuels, fuel cells, industrial greening ... Using the country’s largest proton exchange membrane (PEM) electrolyser, Plug Power’s Georgia plant now produces 15 ...
- Plug Power, Inc.: Plug Signs MOU with Allied Green Ammonia for 3GW of Electrolyzer Supply at World-Class Green Ammonia Production Facilityon May 3, 2024 at 6:01 am
Our expertise in constructing and operating large-scale hydrogen production facilities and our PEM electrolyzer manufacturing capability ... In creating the first commercially viable market for ...
- Hydrogen Fuel Cell market is projected to grow at a CAGR of 21.7% by 2034: Visiongainon May 3, 2024 at 2:40 am
Visiongain has published a new report entitled Hydrogen Fuel Cell Market Report 2024-2034: Forecasts by Application (Stationary Power Generation, Transportation, Portable Devices, Other), by Type ...
- Plug Signs MOU with Allied Green Ammonia for 3GW of Electrolyzer Supply at World-Class Green Ammonia Production Facilityon May 3, 2024 at 12:00 am
Our expertise in constructing and operating large-scale hydrogen production facilities and our PEM electrolyzer manufacturing capability ... In creating the first commercially viable market for ...
- BWR Innovations Chosen to Deliver PEM Fuel Cells for Hawaii Base Microgrid H2 Integrationon May 2, 2024 at 2:08 pm
Global Connective Center LLC granted BWR Innovations the two-year subcontract as part of an agreement with the Air Force Research Laboratory (AFRL). The Hydrogen Fuel Cell ...
- Toyota goes large on hydrogen with new US headquarterson May 1, 2024 at 10:34 am
Toyota today announced that it's turning its R&D office in Los Angeles into its new North American hydrogen headquarters.
- The future of clean hydrogenon April 16, 2024 at 5:01 pm
presented results on the usability of Nafion™ membranes for PEM electrolysis. Barry Sharpe, Vice President of Operations and General Manager of the Bloom Manufacturing Center of Delaware, provided an ...
- IDTechEx Discusses Membrane Materials for PEM Fuel Cells in the Post-PFAS Worldon April 16, 2024 at 1:36 am
The IDTechEx report on the topic, "Materials for PEM Fuel Cells 2024-2034: Technologies, Markets, Players", gives a detailed technical overview of membranes and other key components for PEM fuel ...
- IDTechEx Discusses Membrane Materials for PEM Fuel Cells in the Post-PFAS Worldon April 15, 2024 at 5:00 pm
BOSTON, April 16, 2024 /PRNewswire/ -- Membrane materials are of fundamental importance for fuel cells, even giving their name to the specific type of fuel cell, as is the case for Proton Exchange ...
- Fuel cells: Oxidation processes of phosphorous acid revealed by tender X-rayson April 2, 2024 at 4:59 pm
In addition, the results also point to possible improvements of the operating conditions of HT-PEM fuel cells, e.g. by controlling the humidification to oxidize the H 3 PO 3 back to H 3 PO 4.
via Bing News