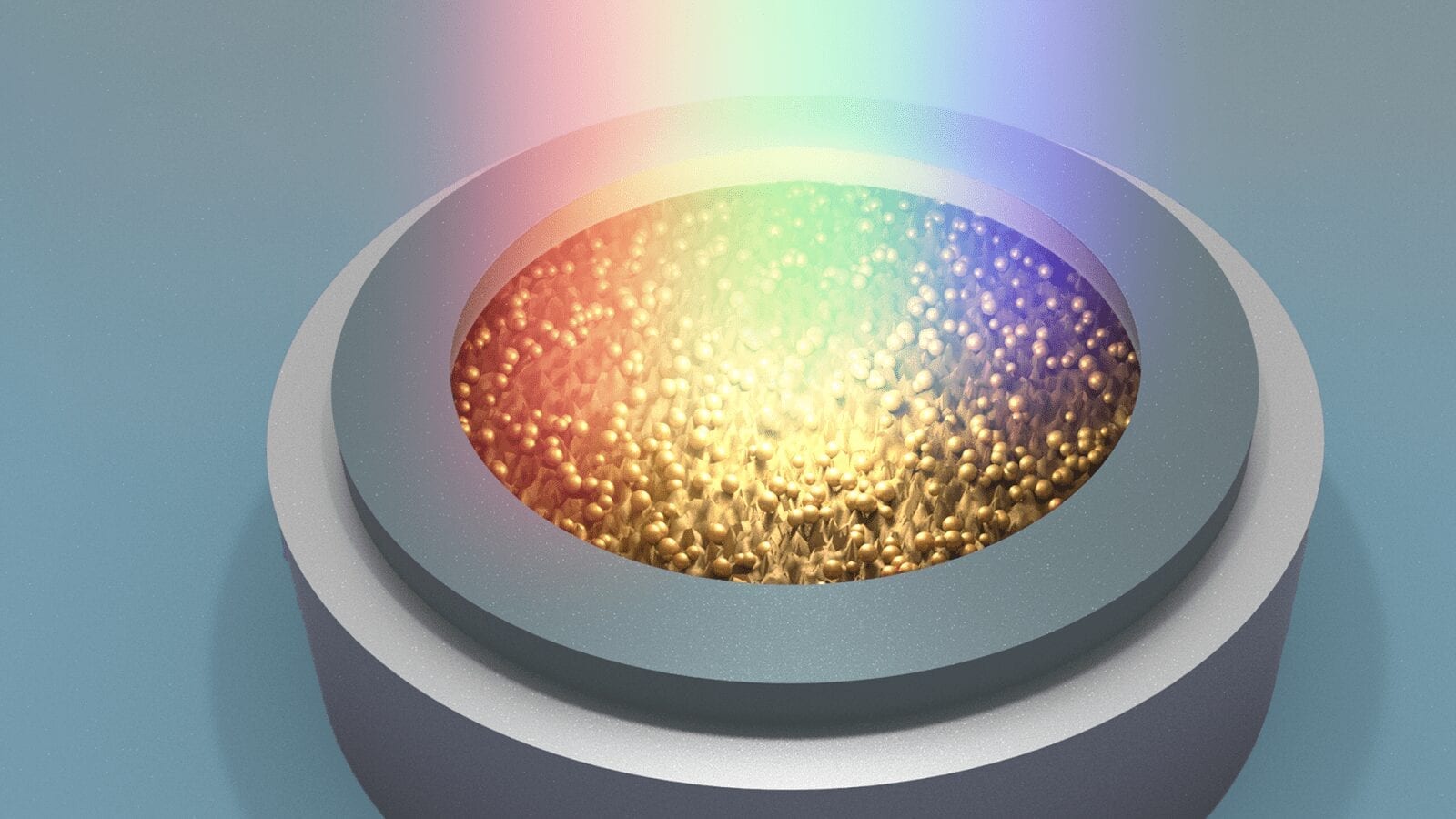
Exposing cathodes to light decreases charge time by a factor of two in lithium-ion batteries.
Researchers at the U.S. Department of Energy’s (DOE) Argonne National Laboratory have reported a new mechanism to speed up the charging of lithium-ion batteries for electric vehicles. Simply exposing the cathode to a beam of concentrated light — for example, the white light from a xenon lamp — lowers the battery charging time by a remarkable factor of two or more. If commercialized, such technology could be a game changer for electric vehicles.
Owners of electric vehicles are well aware of “range anxiety” as the charge level runs low or the location of the closest charging station seems too distant. Fast charging remains a critical challenge if such vehicles are ever to capture a large segment of the transportation market. Charging for an electric car on empty typically takes about eight hours.
“We wanted to greatly shorten this charge reaction without damaging the electrodes from the resulting higher current flow.” — Christopher Johnson, Argonne Distinguished Fellow
Special supercharging stations now exist that achieve ultrafast charging of electric vehicles by delivering a much higher current to the battery. Passing too much current over too short a time, however, degrades battery performance.
Typically, lithium-ion batteries for vehicles are slowly charged to obtain a complete electrochemical reaction. This reaction involves removing lithium from the oxide cathode and inserting it into the graphite anode.
“We wanted to greatly shorten this charge reaction without damaging the electrodes from the resulting higher current flow,” said Christopher Johnson, Argonne Distinguished Fellow and group leader in the Chemical Sciences and Engineering division.
Today’s lithium-ion batteries work in a dark state, with the electrodes housed in a case. Argonne’s photo-assisted technology would use a transparent container that allows concentrated light to illuminate the battery electrodes during charging.
To probe the charge process, the research team crafted small lithium-ion cells (“coin cells”) with transparent quartz windows. They then tested these cells with and without white light shining through the window onto the cathode.
?“We hypothesized that, during charging, white light would interact favorably with the typical cathode material, and that proved to be the case in our cell tests,” Johnson said. That cathode material is a lithium manganese oxide, abbreviated as LiMn2O4 (LMO).
The key ingredient in this favorable reaction is the interplay of light with LMO, a semiconducting material known to interact with light. While absorbing the photons in the light during charging, the element manganese in the LMO changes its charge state from trivalent to tetravalent (Mn3+ to Mn4+). In response, lithium ions eject faster from the cathode than would occur without the photon-excitation process.
This condition drives the battery reaction faster. The team found that the faster reaction resulted in faster charging without degrading battery performance or cycle life. ?“Our cell tests showed a factor of two decrease in charging time with the light turned on,” Johnson said.
Learn more: Shedding new light on the charging of lithium-ion batteries
The Latest on: Battery charging
[google_news title=”” keyword=”battery charging” num_posts=”10″ blurb_length=”0″ show_thumb=”left”]
via Google News
The Latest on: Battery charging
- Sheffield e-bike house fire - Sheffield e-bike house fire - BBC Soundson April 29, 2024 at 4:49 am
Three people taken to hospital after house fire caused by charging e-bike battery. Programme Website. Coming Up Next. now playing
- Tesla EVs could get a massive range boost from new battery tech that promises a 373-mile range from a 10-minute chargeon April 29, 2024 at 4:28 am
Chinese battery-maker CATL announces its newest technology that boasts a massive range and fast recharge times.
via Bing News