A transition to hydrogen as a major fuel in the next 50 years could fundamentally transform the U.S. energy system, according to the National Research Council, and a surprise discovery at New Mexico State University may speed that transformation.
David Johnson was a doctoral candidate in 2007 working on lab experiments for his dissertation in molecular biology at NMSU when he unexpectedly identified a biopolymer that could result in safer hydrogen storage.
Johnson, who now has his doctorate and is working at the NMSU Institute for Energy and Environment, was working on a National Science Foundation-funded grant to find a unique hydrogen-producing microbial community when he noticed some unusual bubbles forming in the reactor.
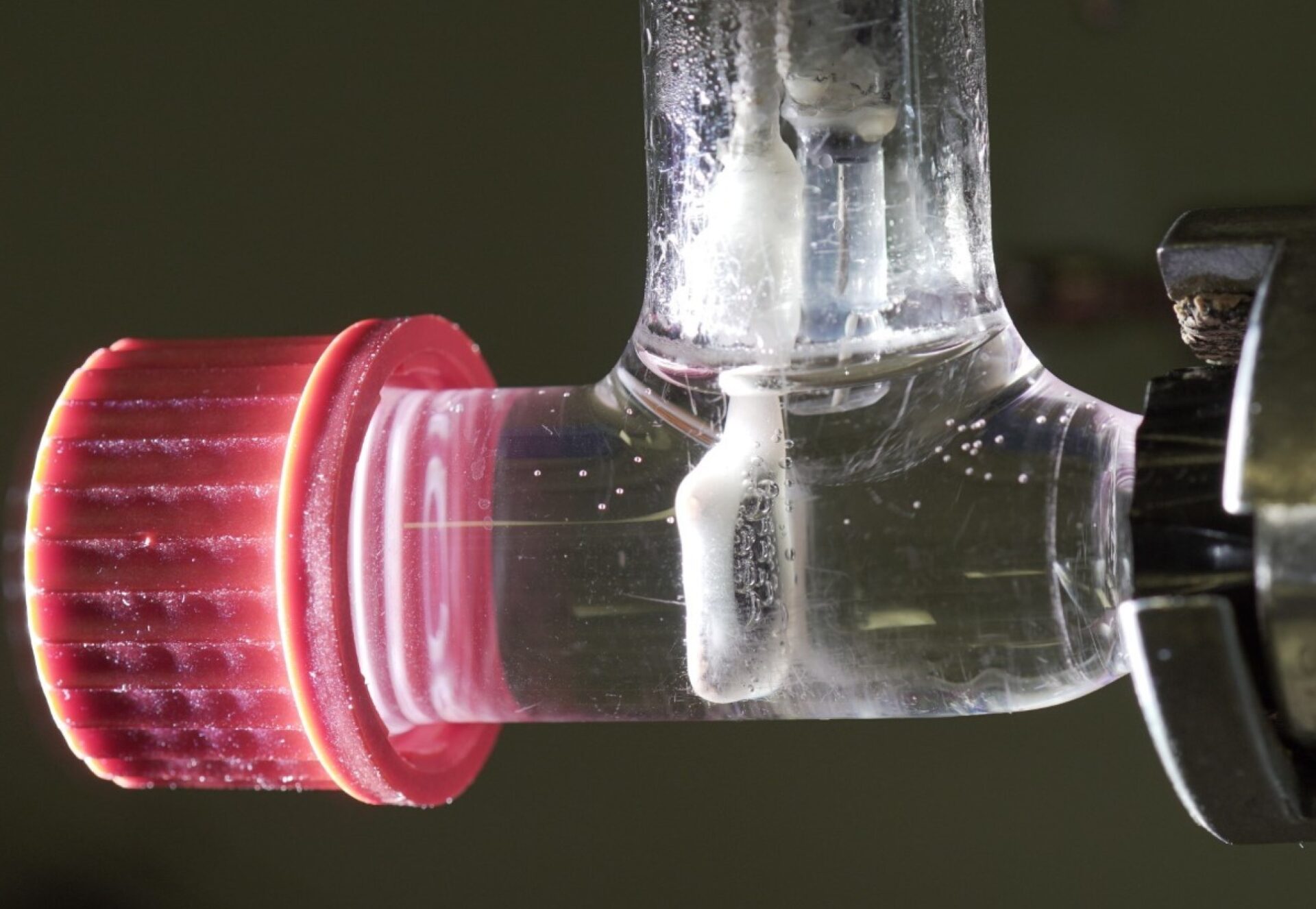
The bright white bubbles were forming a biopolymer that NMSU biology researcher and professor Geoffrey Smith didn’t recognize.
This biopolymer was also capturing and storing hydrogen as the gas was being produced. With a tight molecular structure that made it impermeable to hydrogen, the biopolymer also demonstrated elastomeric properties that are similar to natural rubber.
Smith screened the polymer against thousands of known compounds, but it wasn’t clear what organisms produced the polymer.
“We then sampled the polymer bubble with a gas-tight syringe and injected samples into a gas chromatograph,” Smith said. “We found hydrogen gas, along with some other gases, in the bubbles.”
Hydrogen, the smallest molecule in the universe, is hard to contain because of its size and corrosive properties. Expensive PVC piping and stainless steel materials are currently being used for hydrogen storage and transport, but hydrogen diffusion erodes metals over time, causing them to become brittle. This leads to structural failures in storage and transporting piping.
The polymer’s properties encouraged Smith to think of potential applications.
In 2009, through NMSU’s Launch proof-of-concept program at Arrowhead Center, Smith connected with Michael Townsand, a master’s biology lab researcher. Townsand reviewed Johnson’s dissertation notes to try and replicate the serendipitous discovery.
Townsand proved that the polymer producer was a yeast, and was able to replicate the biopolymer production after simplifying the production feeding the yeast sugar under specific temperature and pH conditions in a process that came to be termed “biohydrogenesis.”
“The enzymes inside of the cell are the essence of biotechnology,” Smith said, “which is harnessing natural processes that synthesize and export the polymer outside the cell.”
To protect the technology, Smith turned to Arrowhead Center’s Office of Intellectual Property and Technology Transfer, and has applied for a patent for the polymer, now known as Hydromer. Arrowhead’s director of intellectual property and technology transfer, Terry Lombard, is working with Smith on commercializing Hydromer as a coating material will allow for low maintenance costs and high durability for hydrogen storage.
“Dr. Smith’s Hydromer technology is expected to provide a safe hydrogen storage method that may overcome some of the existing concerns with hydrogen transport,” Lombard said, “and move the trend and experiments with hydrogen-fueled vehicles quicker to commercialization and the marketplace.”
Read more: Accidental Discovery Bubbles with Promise for Safer Hydrogen Storage
The Latest on: Hydrogen Storage
[google_news title=”” keyword=”Hydrogen Storage” num_posts=”10″ blurb_length=”0″ show_thumb=”left”]
via Google News
The Latest on: Hydrogen Storage
- IPCL and ES2 collaborate for pilot thermal energy storage projecton May 8, 2024 at 9:57 pm
The two companies launched a thermal storage unit with a 250 KWh capacity. Indian Power Corporation Limited (IPCL) and ES2 Power signed an agreement to launch the 250-kilowatt-hour pilot thermal ...
- Why Attending the 2024 Hydrogen Technology Expo is Essentialon May 8, 2024 at 9:43 am
Houston, Texas, is poised to become the focal point of the hydrogen and clean energy industry as it prepares to host the Hydrogen Technology Expo North America ...
- Hydrogen Storage Alloy Market Forecast 2024-2034: NiMH Alloys and Nickel Hydride Batteries Hold Significant Market Shareon May 7, 2024 at 9:32 pm
Dublin, May 08, 2024 (GLOBE NEWSWIRE) -- The "Hydrogen Storage Alloy Market - A Global and Regional Analysis: Focus on Application, Type, and Region - Analysis and Forecast, 2024-2034" report has been ...
- Powering homes exclusively with hydrogen, solar, batterieson May 7, 2024 at 9:10 am
Researchers in Spain has found that combining PV power generation with fuel cells and battery storage may help homes considerably reduce their levelized cost of energy. Their simulation reportedly ...
- Feadship Launches World’s First Hydrogen Fuel Cell-Powered Superyachton May 6, 2024 at 3:08 pm
This new, 390-foot-long superyacht (designed by RWD with owner’s representation by Edmiston), can provide carbon-emission-free power from green ...
- Researchers propose use of cesium, rubidium for hydrogen batterieson May 6, 2024 at 9:28 am
A study led by Russia’s Skoltech and China’s HPSTAR suggests that rubidium and cesium additives could improve the efficiency of hydrogen batteries. Researcher Dmitrii Semenok tells pv magazine that ...
- This New Development Shows Toyota’s Unwavering Commitment Towards Hydrogen Fuel Cell Technologyon May 1, 2024 at 6:45 am
In a bid to stay ahead of the hydrogen-tech curve,Toyota has decided to build a Hydrogen Headquarters to accelerate the advancement of fuel cell tech.
- Scientist hails commercial feasibility of Saudi Arabian hydrogen city planon April 30, 2024 at 7:35 am
Alberto Boretti was a senior research professor at Prince Mohammad Bin Fahd University in 2021 when he first started discussing the idea of a hydrogen city in Al Khobar, Saudi Arabia. The New ...
- Subterranean storage of hydrogenon April 9, 2024 at 2:59 pm
"Hydrogen would be good for seasonal and long-term storage," said Sandia chemical engineer Tuan Ho, who is leading the research. "If you think of solar energy, in the summer you can produce a lot ...
- Sandia studies subterranean storage of hydrogenon April 9, 2024 at 7:56 am
“Hydrogen would be good for seasonal and long-term storage,” said Sandia chemical engineer Tuan Ho, who is leading the research. “If you think of solar energy, in the summer you can produce ...
via Bing News