Osaka University researchers develop efficient “green” hydrogen production system that operates at room temperature in air.
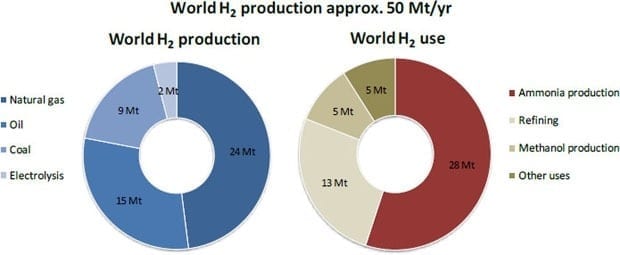
Hydrogen gas is a promising alternative energy source to overcome our reliance on carbon-based fuels, and has the benefit of producing only water when it is reacted with oxygen. However, hydrogen is highly reactive and flammable, so it requires careful handling and storage. Typical hydrogen storage materials are limited by factors like water sensitivity, risk of explosion, difficulty of control of hydrogen-generation. Hydrogen gas can be produced efficiently from organosilanes, some of which are suitably air-stable, non-toxic, and cheap. Catalysts that can efficiently produce hydrogen from organosilanes are therefore desired with the ultimate goal of realizing safe, inexpensive hydrogen production in high yield. Ideally, the catalyst should also operate at room temperature under aerobic conditions without the need for additional energy input.
A research team led by Kiyotomi Kaneda and Takato Mitsudome at Osaka University have now developed a catalyst that realizes efficient environmentally friendly hydrogen production from organosilanes. The catalyst is composed of gold nanoparticles with a diameter of around 2 nm supported on hydroxyapatite. The catalyst was synthesized from chloroauric acid using glutathione as a capping agent to prevent nanoparticle aggregation, resulting the formation of small size of gold nanoparticles. Glutathione-capped gold nanoparticles were then adsorbed on hydroxyapatite and glutathione was removed by subsequent calcination.
The team then added the nanoparticle catalyst to solutions of different organosilanes to measure its ability to induce hydrogen production. The nanoparticle catalyst displayed the highest turnover frequency and number attained to date for hydrogen production catalysts from organosilanes. For example, the nanoparticle catalyst converted 99% of dimethylphenylsilane to the corresponding silanol in just 9 min at room temperature, releasing an equimolar amount of hydrogen gas at the same time. Importantly, the catalyst was recyclable without loss of activity. On/off switching of hydrogen production was achieved using the nanoparticle catalyst because it could be easily separated from its organosilane substrate by filtration. The activity of the catalyst increased as the nanoparticle size decreased.
A prototype portable hydrogen fuel cell containing the nanoparticle catalyst and an organosilane substrate was fabricated. The fuel cell generated power in air at room temperature and could be switched on and off as desired. Images of the catalyst after use in the fuel cell resembled those of the unused catalyst, indicating that the hydroxyapatite-supported nanoparticle catalyst readily resisted aggregation.
Generation of hydrogen from inexpensive organosilane substrates under ambient conditions without additional energy input represents an exciting advance towards the goal of using hydrogen as a green energy source.
Learn more: Safe and Inexpensive Hydrogen Production as a Future Energy Source
[osd_subscribe categories=’hydrogen-production’ placeholder=’Email Address’ button_text=’Subscribe Now for any new posts on the topic “HYDROGEN PRODUCTION”‘]
Receive an email update when we add a new HYDROGEN PRODUCTION article.
The Latest on: Hydrogen production
[google_news title=”” keyword=”hydrogen production” num_posts=”10″ blurb_length=”0″ show_thumb=”left”]
via Google News
The Latest on: Hydrogen production
- Mitsubishi Heavy Industries begins testing solid oxide electrolyzer cell for hydrogen productionon April 26, 2024 at 6:01 pm
The SOEC test module includes multiple cartridges of 500 cell stacks bundled together. MHI said results will be used to support higher output and capacity.
- California Welcomes First Big-Rig Hydrogen Fuel Station in U.S.on April 26, 2024 at 9:08 am
The country’s first commercial hydrogen fuel station for big-rig trucks is up and running at the Port of Oakland, a step toward what hydrogen proponents see as a clean new future for long-haul ...
- Custom-made catalyst leads to longer-lasting and more sustainable green hydrogen productionon April 26, 2024 at 8:50 am
Researchers led by Ryuhei Nakamura at the RIKEN Center for Sustainable Resource Science (CSRS) in Japan have improved on their green and sustainable method of extracting hydrogen from water by using a ...
- The first big-rig hydrogen fuel station in the US opens in Californiaon April 26, 2024 at 4:39 am
Making hydrogen itself is now a dirty, greenhouse-gas-generating process, although green hydrogen production is an emerging option, and even more expensive. Hydrogen proponents are banking on the idea ...
- Metal swarf transformed into electrodes for hydrogen productionon April 26, 2024 at 3:39 am
Nanotextured surface of titanium and nickel waste supports platinum and cobalt atoms to create effective electrocatalysts ...
- SJVN unveils India's 1st multi-purpose green hydrogen projecton April 25, 2024 at 9:35 pm
The hydrogen production will be powered using solar power. SJVN has launched India’s first multi-purpose green hydrogen project which is a combined heat and power located in Jhakri, Himachal Pradesh.
- Nikola’s Hydrogen-Truck Fueling Strategyon April 25, 2024 at 5:00 pm
One of the criticisms of hydrogen as a transport fuel has been that the production of hydrogen itself can be a source of pollution and greenhouse gases. And that is something that Hyla is working to ...
- 3 Hydrogen Stocks That Could Be Multibaggers in the Making: April Editionon April 25, 2024 at 3:15 pm
InvestorPlace - Stock Market News, Stock Advice & Trading Tips There are some multibagger hydrogen stocks that investors should have on their ...
- 3 Hydrogen Stocks to Buy on the Dip: April 2024on April 25, 2024 at 3:04 pm
InvestorPlace - Stock Market News, Stock Advice & Trading Tips Keep an eye on hydrogen stocks to buy on the dip. For one, according ...
via Bing News