A University of Adelaide-led research team has developed a new approach to purging water sources of the microplastics that pollute them without harming nearby microorganisms.
Plastic waste finds its way into oceans and rivers poses a global environmental threat with damaging health consequences for animals, humans, and ecosystems.
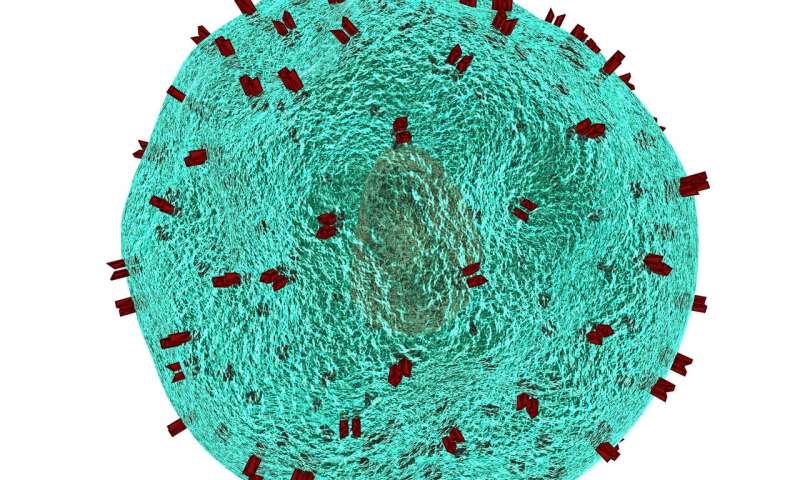
The researchers have developed a technique to break down the microplastics using tiny coil-shaped carbon-based magnets. Their work is published in the journal Matter.
“Microplastics adsorb organic and metal contaminants as they travel through water and release these hazardous substances into aquatic organisms when eaten, causing them to accumulate all the way up the food chain,” says senior author Shaobin Wang, Professor of Chemical Engineering at the University of Adelaide. “Carbon nanosprings are strong and stable enough to break these microplastics down into compounds that do not pose such a threat to the marine ecosystem.”
Although often invisible to the naked eye, microplastics are ubiquitous pollutants. Some, such as the exfoliating beads found in popular cosmetics, are simply too small to be filtered out during industrial water treatment. Others are produced indirectly, when larger debris like soda bottles or tires weather amid sun and sand.
The research is a collaboration between University of Adelaide, Curtin University, Edith Cowan University and Guangdong University of Technology in China.
To decompose the microplastics, the researchers had to generate short-lived chemicals called reactive oxygen species, which trigger chain reactions that chop the various long molecules that make up microplastics into tiny and harmless segments that dissolve in water. However, reactive oxygen species are often produced using heavy metals such as iron or cobalt, which are dangerous pollutants in their own right and thus unsuitable in an environmental context.
To get around this challenge, the researchers found a greener solution in the form of carbon nanotubes laced with nitrogen to help boost generation of reactive oxygen species. Shaped like springs, the carbon nanotube catalysts removed a significant fraction of microplastics in just eight hours while remaining stable themselves in the harsh oxidative conditions needed for microplastics breakdown. The coiled shape increases stability and maximises reactive surface area. As a bonus, by including a small amount of manganese buried far from the surface of the nanotubes to prevent it from leaching into water, the minute springs became magnetic.
“Having magnetic nanotubes is particularly exciting because this makes it easy to collect them from real wastewater streams for repeated use in environmental remediation,” says project co-leader Dr Xiaoguang Duan, Research Fellow in the University of Adelaide’s School of Chemical Engineering and Advanced Materials.
As no two microplastics are chemically quite the same, the researchers’ next steps will centre on ensuring that the nanosprings work on microplastics of different compositions, shapes and origins. They also intend to continue to rigorously confirm the non-toxicity of any chemical compounds occurring as intermediates or by-products during microplastics decomposition.
The researchers also say that those intermediates and byproducts could be harnessed as an energy source for microorganisms that the polluting plastics currently plague. “If plastic contaminants can be repurposed as food for algae growth, it will be a triumph for using biotechnology to solve environmental problems in ways that are both green and cost efficient,” Professor Wang says.
Learn more: BREAKING DOWN MARINE PLASTIC POLLUTION
The Latest on: Microplastics
[google_news title=”” keyword=”microplastics ” num_posts=”10″ blurb_length=”0″ show_thumb=”left”]
via Google News
The Latest on: Microplastics
- Swarms of miniature robots clean up microplastics and microbes, simultaneouslyon May 8, 2024 at 8:14 am
When old food packaging, discarded children's toys and other mismanaged plastic waste break down into microplastics, they become even harder to clean up from oceans and waterways. These tiny bits of ...
- America has a $250 billion problem: Microplastics have invaded our bloodstreams and may increase the risk of heart attack and strokeon May 8, 2024 at 7:52 am
For all the damage that microplastics are doing to the planet, it may be that only an impending threat to the human body will direct the kind of attention to the issue that it has long deserved. That ...
- Magnetic microrobot swarms clean water of microplastics and bacteriaon May 8, 2024 at 6:02 am
Microplastics are one of the biggest environmental and health risks faced by our generation. Now, researchers have developed swarms of tiny robots, or microrobots, that not only microplastics from ...
- Swarms of miniature robots clean up microplastics and microbes, simultaneously (video)on May 8, 2024 at 5:31 am
Swarms of magnetically controlled microrobots removed both microplastics and microbes from water. And the bots could be decontaminated and reused. This is a promising approach for decontaminating ...
- Video shows how swarms of miniature robots simultaneously clean up microplastics and microbeson May 8, 2024 at 5:00 am
When old food packaging, discarded children's toys and other mismanaged plastic waste break down into microplastics, they become even harder to clean up from oceans and waterways. These tiny bits of ...
- Microplastics are everywhere, even in your bloodstreamon May 7, 2024 at 2:15 am
As the film unravels, audiences are made scarily aware of the presence of microplastics in food, soil, clothes, blood, feces and daily household items. More shockingly, the film captures microplastics ...
- 'Everywhere we looked, we found evidence': Microplastics expert on 20 years of pollution researchon May 6, 2024 at 9:14 am
Thirty years ago, while counting barnacles, limpets and seaweeds along rocky shores, I started noticing a daily tide of litter, mostly plastic. As a marine biology Ph.D. student at Liverpool ...
- Microplastics: A looming threat to our well-beingon May 1, 2024 at 3:17 am
The pervasive presence of microplastics in our environment has become an issue of global concern, prompting urgent action from scientists, policymakers, and individuals alike. These tiny plastic ...
- Toxic chemicals from microplastics can be absorbed by the skin, study findson April 26, 2024 at 8:20 am
A new study used 3D human skin-equivalent models to examine how flame retardant additives in microplastics are absorbed by the skin. The findings show that several flame-retardant additives passed ...
- What are microplastics doing to human health? Scientists work to connect the dotson April 26, 2024 at 1:01 am
People unknowingly ingest microplastics from what we eat, drink and breathe. Some scientists fear exposure to microplastics could increase vulnerability to heart disease, cancer and other illnesses.
via Bing News