A University of Arkansas for Medical Sciences (UAMS) research team led by Vladimir Zharov, Ph.D., D.Sc., has demonstrated the ability to detect and kill circulating tumor cells (CTCs) in the blood using a noninvasive device called Cytophone that integrates a laser, ultrasound and phone technologies.
This device is 1,000 times more sensitive than other methods at detecting of CTCs in the blood of patients with melanoma, a deadly form of skin cancer.
The Cytophone also has shown the ability to detect CTCs even when the tumor is not identifiable on the skin, either because too small (known as the T0 or TX stage) or after surgical removal, and then to destroy them without harming surrounding blood cells.
The team’s findings titled “In Vivo Liquid Biopsy using Cytophone Platform for Photoacoustic Detection of Circulating Tumor Cells in Melanoma Patients” were published in the June 12 issue of Science Translational Medicine, a prestigious scientific journal published by the American Association for the Advancement of Science.
“The only methods that are available to detect CTCs are mainly based on drawing blood from the patient. An average blood sample taken from a patient consists of only a few milliliters, which may or may not contain any CTCs. In contrast, the Cytophone can monitor a person’s entire five-liter blood supply, potentially locating every CTC in it. No needle is used, and no blood is removed,” said Zharov, a professor in the UAMS College of Medicine Department of Otolarynology-Head and Neck Surgery.
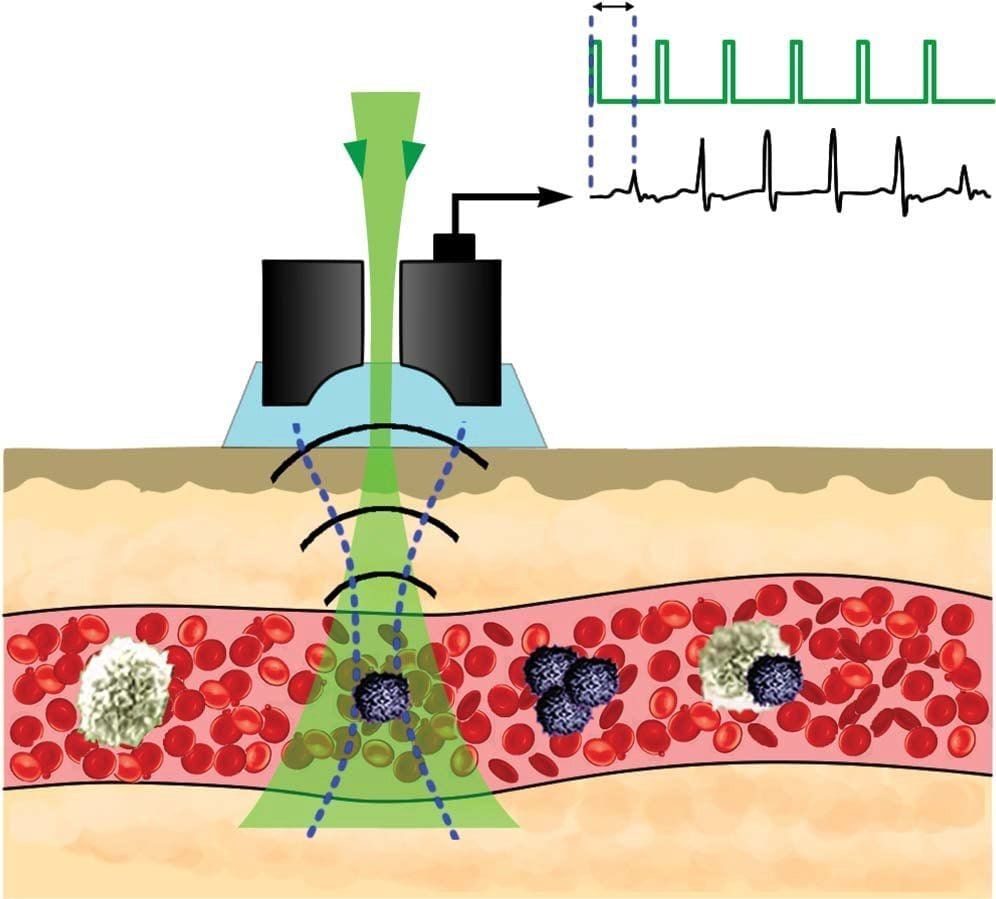
The portable Cytophone platform is based on a principle called in vivo photoacoustic flow cytometry, a technology that uses laser pulses to penetrate through intact skin and into blood vessels to monitor circulating abnormal cells and other disease-associated biomarkers. The published research describes the first demonstration of noninvasive detection of CTCs directly in the bloodstream of melanoma patients. When a melanoma CTC passes the laser beam into the vessels, the laser pulses heat the natural melanin nanoparticles in these cells. The fast thermal expansion of these nanoparticles then generates a sound that is detected using an ultrasound transducer attached to the skin.
The research team demonstrated that the advanced software with fast signal processing algorithms makes Cytophone data tolerant to skin pigmentation and motion that led to the identification of CTCs in 96% of the patients in between 10 seconds and 60 minutes without generating false positives in the controls at the established thresholds and current detection limit of five CTCs in five liters of blood.
Researchers found that the device not only discovered CTCs in advanced stage patients, but also revealed the presence of CTCs in the patients with early Stage 2 disease.
“With the Cytophone, we can listen to the laser-triggering sound from each individual cell in the body. CTCs could be one of the best early metastasis markers, because obviously only viable CTCs can create deadly spread of the disease,” said Zharov, who also serves as director of the Arkansas Nanomedicine Center and holds the Josephine T. McGill Chair in Cancer Research at UAMS.
This technology also has demonstrated the ability to destroy the detected CTCs, resulting in a large drop in CTC numbers and preventing spread of the disease to other parts of the body, known as metastasis.
Thus, the Cytophone may be able to serve as a theranostic platform by integrating both diagnostic and therapeutic capabilities using the same laser to detect and kill the cancer cells right in the bloodstream.
The Zharov team also demonstrated the Cytophone performance for the identification of cancer-related blood clots, which is the second leading cause of death among cancer patients.
“We are developing the robust, easy-to-use portable and wearable Cytophone versions with advanced small lasers, which will be available for cancer clinics across the country to start a multi-center clinical trial involving more melanoma patients. Our goal is to determine whether Cytophone-based early diagnosis combined with destroying CTCs is effective as a stand-alone treatment or in combination with conventional therapies in preventing or at least inhibiting metastasis,” said Zharov.
Zharov’s team first also demonstrated the ability to detect non-pigmented CTCs by injecting a cocktail of magnetic and gold nanoparticles with a special biological coating into the bloodstream. Breast cancer-related clinical trials are in progress that takes into account successful preclinical trials using this technology previously published in the journal Nature Nanotechnology.
Other applications for the Cytophone in label-free mode could include detection of sickle cells to prevent sickle cell crisis, detection of clots to prevent stroke, and selection of the most effective drug through monitoring of circulating disease-associated markers count decrease.
Learn more: UAMS-Developed Cytophone Detects Melanoma in Earliest Stages, Could Prevent Fatal Disease Spread
The Latest on: In vivo photoacoustic flow cytometry
[google_news title=”” keyword=”in vivo photoacoustic flow cytometry” num_posts=”10″ blurb_length=”0″ show_thumb=”left”]
via Google News
The Latest on: In vivo photoacoustic flow cytometry
- Flow Cytometry – Webinars and Online Eventson April 27, 2024 at 11:55 pm
We will discuss automated alternatives to flow-sorting and manual colony picking with that improve single-cell outgrowth and increase gene editing efficiency.
- We need to talk about flow cytometryon November 27, 2023 at 7:53 pm
It may surprise many to hear that some of today’s most advanced treatments, such as cell therapies for cancer, diabetes, and autoimmunity, depend on flow cytometry — a 50-year old technology.
- Flow Cytometryon August 16, 2023 at 4:58 am
Flow cytometry has inarguable potential as a clinical tool for disease diagnosis, prognosis, and therapeutic monitoring. However, some challenges remain in translating the full promise of FCM into ...
- Flow Cytometry Research Core Facilityon July 31, 2023 at 2:30 am
Established in 1995, the Flow Cytometry Research Core Facility serves as an integral part of numerous research projects for investigators from multiple departments and disciplines at Saint Louis ...
- Flow cytometry core serviceon March 3, 2023 at 1:35 pm
The Medical School's flow cytometry facility is a core facility based in the RHH Medical School and provides a service to all at the University and externally. Flow cytometry is a technique that ...
- Cytometry and Cell Sorting Coreon October 20, 2021 at 11:08 am
The Cytometry and Cell Sorting Facility at Baylor College of Medicine provides training, instrumentation, technical expertise, and software for flow cytometric analysis and cell sorting. This site ...
- Advances in flow cytometry enable new cancer researchon May 20, 2021 at 1:38 pm
Flow cytometry has long enabled basic and clinical research, with applications in fields as diverse as molecular biology, neuroscience and plant and marine biology. Recently, its use in cancer ...
- Biomedicum Flow Cytometryon February 25, 2021 at 4:06 am
The instrument has five lasers: Ultra-violet (349nm), Violet (405nm), Blue (488 nm), Yellow-Green (561nm) and Red (638nm) with spectral detectors and 3 imaging optics from blue laser with multiple ...
- Flow Cytometryon January 19, 2021 at 6:16 pm
The Flow Cytometry shared resource stays at the leading edge of technology. Close ties to industry allow us to offer users early access to new reagents and instrumentation. We help users realize a ...
- Flow Cytometryon August 13, 2020 at 4:17 pm
Flow cytometry uses fluorescent probes to identify and characterize cells or particles in suspension (e.g. cells, nuclei or chromosomes) by virtue of size, granularity and fluorescence ...
via Bing News