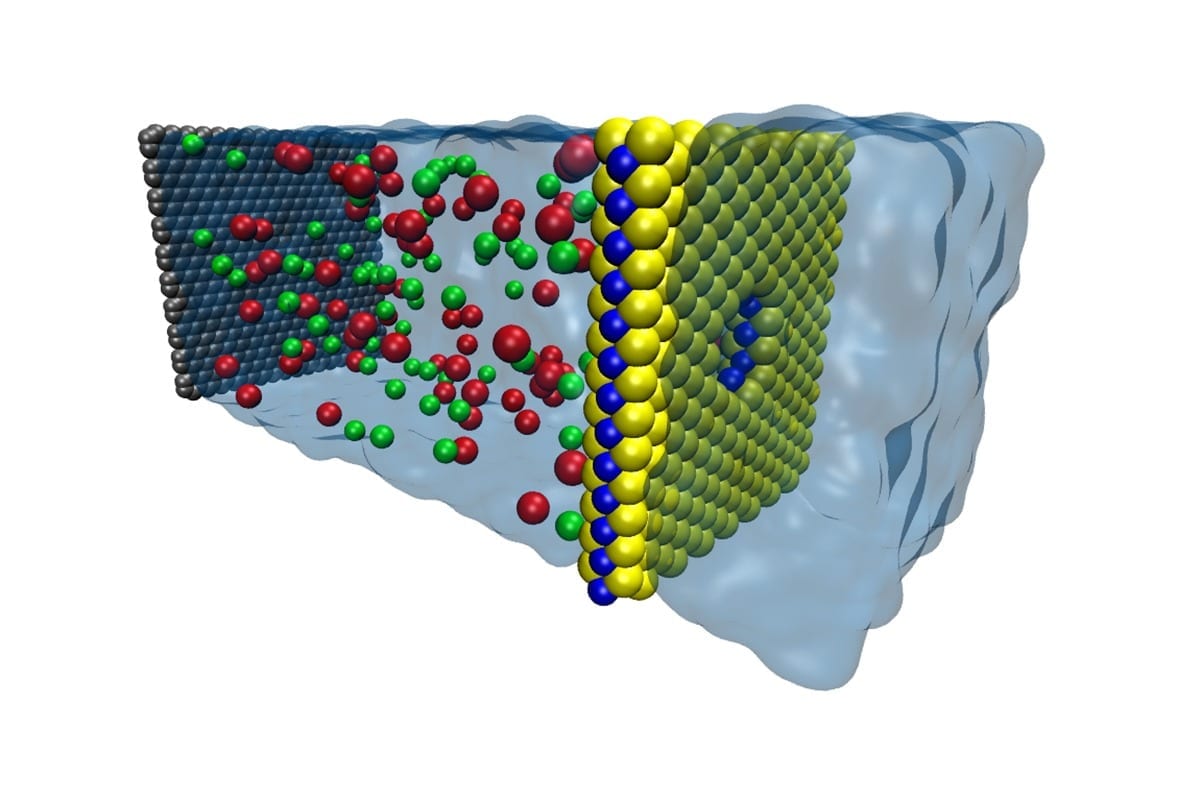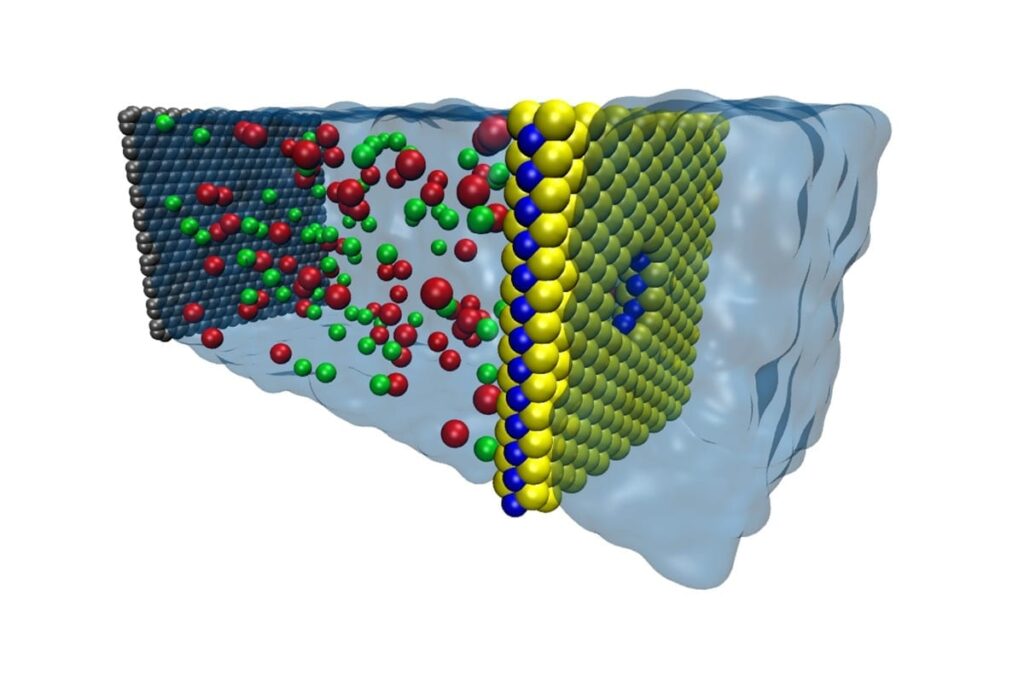
University of Illinois engineers have found an energy-efficient material for removing salt from seawater that could provide a rebuttal to poet Samuel Taylor Coleridge’s lament, “Water, water, every where, nor any drop to drink.”
The material, a nanometer-thick sheet of molybdenum disulfide (MoS2) riddled with tiny holes called nanopores, is specially designed to let high volumes of water through but keep salt and other contaminates out, a process called desalination. In a study published in the journal Nature Communications, the Illinois team modeled various thin-film membranes and found that MoS2 showed the greatest efficiency, filtering through up to 70 percent more water than graphene membranes.
“Even though we have a lot of water on this planet, there is very little that is drinkable,” said study leader Narayana Aluru, a U. of I. professor of mechanical science and engineering. “If we could find a low-cost, efficient way to purify sea water, we would be making good strides in solving the water crisis.
“Finding materials for efficient desalination has been a big issue, and I think this work lays the foundation for next-generation materials. These materials are efficient in terms of energy usage and fouling, which are issues that have plagued desalination technology for a long time,” said Aluru, who also is affiliated with the Beckman Institute for Advanced Science and Technologyat the U. of I.
Most available desalination technologies rely on a process called reverse osmosis to push seawater through a thin plastic membrane to make fresh water. The membrane has holes in it small enough to not let salt or dirt through, but large enough to let water through. They are very good at filtering out salt, but yield only a trickle of fresh water. Although thin to the eye, these membranes are still relatively thick for filtering on the molecular level, so a lot of pressure has to be applied to push the water through.
“Reverse osmosis is a very expensive process,” Aluru said. “It’s very energy intensive. A lot of power is required to do this process, and it’s not very efficient. In addition, the membranes fail because of clogging. So we’d like to make it cheaper and make the membranes more efficient so they don’t fail as often. We also don’t want to have to use a lot of pressure to get a high flow rate of water.”
One way to dramatically increase the water flow is to make the membrane thinner, since the required force is proportional to the membrane thickness. Researchers have been looking at nanometer-thin membranes such as graphene. However, graphene presents its own challenges in the way it interacts with water.
Read more: Nanopores could take the salt out of seawater
The Latest on: Desalination
[google_news title=”” keyword=”desalination” num_posts=”10″ blurb_length=”0″ show_thumb=”left”]
via Google News
The Latest on: Desalination
- Kalba desalination plants continue operating consistentlyon April 26, 2024 at 10:29 am
The Sharjah Electricity, Water, and Gas Authority (SEWA) in Kalba assures its subscribers of the uninterrupted operation of its desalination plants, which maintain high reliability. Specialised ...
- Desalination plant behind scheduleon April 25, 2024 at 3:05 pm
A delay in delivery, damage during transit and fouling issues during testing have pushed the desalination plant completion date into 2025.
- MIT Makes Waves with Light-Powered Water Vapor Revelation, Could Change Climate Science and Solar Desalinationon April 25, 2024 at 2:21 pm
MIT researchers discover that light, not just heat, can cause water to evaporate, possibly impacting climate science and drying technologies.
- Desalination, what are the facts?on April 25, 2024 at 5:01 am
One thing you can count on is that any project, large or small, will attract an organisation or individuals to protest. It’s a way of life for some people. The project for a desalination plant in ...
- TCEQ hears from opponents and supporters of Corpus Christi desalination planon April 24, 2024 at 8:37 am
People in Corpus Christi could be the first in Texas to drink treated seawater. Water scarcity in the state is a growing crisis, and ocean desalination is being touted as a solution. But community ...
- New Desalination Technology Promises Freshwater Cheaper Than Tap Wateron April 23, 2024 at 3:31 pm
In a world where access to clean drinking water is becoming increasingly scarce, a team of engineers from MIT and Shanghai Jiao Tong University in China have developed a game-changing solution. Their ...
- About 100 people came to state officials to talk desalination. Here's what they said.on April 20, 2024 at 1:07 am
A meeting for the public to speak on a proposed desalination plant brought about 100 people to the city's convention center. Tensions were high.
- Contentious TCEQ meeting allows all sides to be heard on seawater desalinationon April 19, 2024 at 11:51 am
TCEQ held a much anticipated public comment meeting at the American Bank Center on Thursday. The City of Corpus Christi's Inner Harbor Seawater Desalination Plant was the subject of discussion.
- Barcelona is banking on a floating desalination plant to fight drought in northeastern Spainon April 19, 2024 at 7:00 am
Local authorities say it is a more economical and environmentally sustainable solution than shipping in water.
- Barcelona to get floating desalination plant to help fight drought in northeastern Spainon April 18, 2024 at 8:06 am
BARCELONA, Spain (AP) — Spain’s drought-stricken region of Catalonia will install a floating desalination plant to help the city of Barcelona guarantee its drinking water supply, regional authorities ...
via Bing News