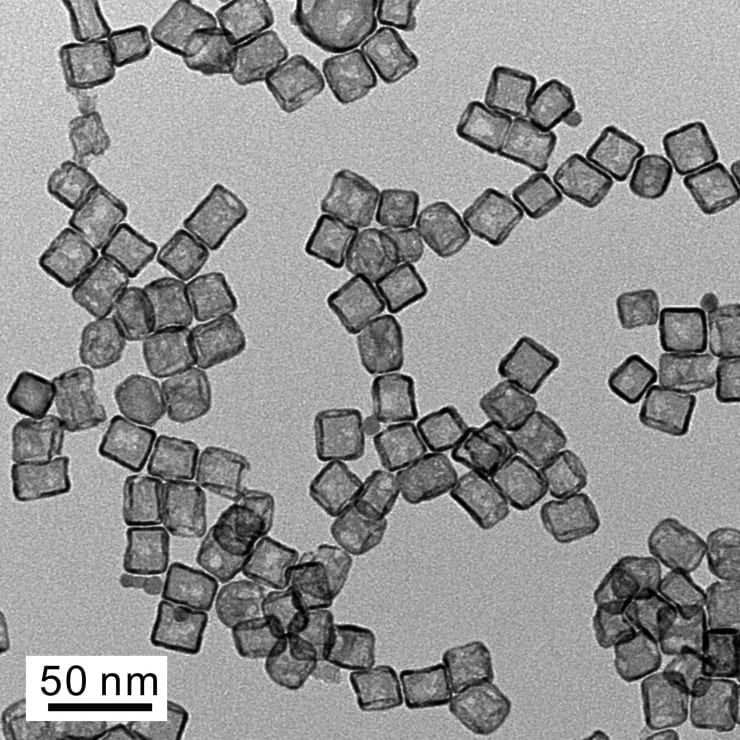
A new fabrication technique that produces platinum hollow nanocages with ultra-thin walls could dramatically reduce the amount of the costly metal needed to provide catalytic activity in such applications as fuel cells.
The technique uses a solution-based method for producing atomic-scale layers of platinum to create hollow, porous structures that can generate catalytic activity both inside and outside the nanocages. The layers are grown on palladium nanocrystal templates, and then the palladium is etched away to leave behind nanocages approximately 20 nanometers in diameter, with between three and six atom-thin layers of platinum.
Use of these nanocage structures in fuel cell electrodes could increase the utilization efficiency of the platinum by a factor of as much as seven, potentially changing the economic viability of the fuel cells.
“We can get the catalytic activity we need by using only a small fraction of the platinum that had been required before,” said Younan Xia, a professor in the Wallace H. Coulter Department of Biomedical Engineering at Georgia Tech and Emory University. Xia also holds joint faculty appointments in the School of Chemistry and Biochemistry and the School of Chemical and Biomolecular Engineering at Georgia Tech. “We have made hollow nanocages of platinum with walls as thin as a few atomic layers because we don’t want to waste any material in the bulk that does not contribute to the catalytic activity.”
The research – which also involved researchers at the University of Wisconsin-Madison, Oak Ridge National Laboratory, Arizona State University and Xiamen University in China – was reported in the July 24 issue of the journal Science.
Platinum is in high demand as a catalyst for a wide range of industrial and consumer applications. The high cost of platinum needed for the catalysts deposited on electrodes has limited the ability to use low-temperature fuel cells in automobiles and home applications.
In catalytic applications, only the surface layers of platinum contribute to the chemical reaction, leading researchers to develop new structures designed to maximize the amount of platinum exposed to reactants. The hollowing out process reduces the amount of the precious metal not contributing to the reaction, and allows the use of larger nanocrystals that are less susceptible to sintering, an aggregation phenomenon which reduces catalyst surface area.
“We can control the process so well that we have layer-by-layer deposition, creating one layer, two layers or three layers of platinum,” said Xia, who is also a Georgia Research Alliance eminent scholar. “We can also control the arrangement of atoms on the surface so their catalytic activity can be engineered to fit different types of reactions.”
Hollow platinum structures have been made before, but not with walls this thin, he added.
Earlier work produced shells with wall thicknesses of approximately five nanometers. The new process can produce shell walls less than one nanometer thick. With both the inner layer and outer layer of the porous nanocages contributing to the catalytic activity, the new structures can use up to two-thirds of the platinum atoms in an ultra-thin three-layer shell. Some palladium remains mixed with the platinum in the structures.
“This approach creates the highest possible surface area from a given amount of platinum,” said Xia.
The nanocages can be made in either cubic or octahedral shapes, depending on the palladium nanocrystals used as templates. The shape controls the surface structure, thus engineering the catalytic activity.
The goal of this research was to reduce the cost of the cathodes in fuel cells designed to power automobiles and homes. The fuel cell’s oxygen-reduction reaction takes place at the cathode, and that requires a substantial amount of platinum. By reducing the amount of platinum by up to a factor of seven, the hollow shells could make automotive and home fuel cells more economically feasible.
The researchers measured the durability of the platinum nanocages for oxygen-reduction reaction, and found the catalytic activity dropped by a little more than one-third after 10,000 operating cycles. Earlier efforts to maximize surface area relied on making very small platinum nanoparticles just two or three nanometers in diameter. Particles of that size tended to clump together in a process known as sintering, reducing the surface area.
“By using hollow structures, we can use much larger particle sizes – about 20 nanometers – and we really don’t lose any surface area because we can use both the inside and outside of the structure, and the shells are only a few atomic layers thick,” Xia added. “We expect the durability of these larger particles to be much better.”
Other applications, such as catalytic converters in automobiles, also use substantial amounts of platinum. The new hollow shells are unlikely to be used in automobile catalytic converters because they operate at a temperature beyond what the structures can tolerate. However, the platinum nanocages could find use in other industrial processes such as hydrogenation.
Contributing to the experimental work done at Georgia Tech, researchers at Arizona State University and Oak Ridge National Laboratory used their specialized microscopy facilities to map the nanocage structures. Researchers at the University of Wisconsin-Madison modeled the system to help understand etching of palladium from the core while preserving the platinum shell.
Researchers have explored alternatives to platinum, but none of the alternatives so far has provided the equivalent amount of catalytic activity in such a small mass, Xia noted.
“If you took all of the platinum that we have available today and made a cube, it would only be seven meters on each side,” he added. “That’s all the platinum we have now, so we need to find the most efficient way to use it.”
Read more: Ultra-thin Hollow Nanocages Could Reduce Platinum Use in Fuel Cell Electrodes
The Latest on: Platinum use
[google_news title=”” keyword=”Platinum use” num_posts=”10″ blurb_length=”0″ show_thumb=”left”]
via Google News
The Latest on: Platinum use
- The Covid-19 Pandemicon May 15, 2024 at 10:05 am
With the acute phase of the Covid-19 pandemic fading even as the coronavirus persists and evolves, a new normal is taking shape around the world. The decision came after a scorching hearing in ...
- Best Antivirus Software for 2024on May 14, 2024 at 2:00 pm
Clifford is a managing editor at CNET, where he leads How-To coverage. He spent a handful of years at Peachpit Press, editing books on everything from the first iPhone to Python. He also worked at ...
- What to know about the bird flu outbreak in the US after virus fragments found in milk sampleson April 25, 2024 at 8:38 am
Several dairy cows have been infected, resulting in milk samples showing inactive remnants of the virus, and one human case has been confirmed. Health officials say the food supply is safe and the ...
- World's chocolate supply threatened by devastating viruson April 22, 2024 at 4:59 pm
A rapidly spreading virus threatens the health of the cacao tree and the dried seeds from which chocolate is made, jeopardizing the global supply of the world's most popular treat. Farmers can ...
- Measles outbreak in Chicago: What to know about the viruson March 27, 2024 at 9:50 am
Though most vaccinated people have close to zero chance of contracting the virus, here’s what to know about the disease. Yes, they do. In fact, measles, which was considered eliminated in the ...
- New study shows virus-like particle can effectively 'shock and kill' latent HIV reservoiron March 26, 2024 at 7:29 am
In the study published in the journal Emerging Microbes and Infections, the team demonstrated that the patented therapeutic candidate, an HIV-virus-like-particle (HLP), is 100 times more effective ...
- Inside the Coronaviruson February 3, 2024 at 9:56 am
We show how the immune system would normally attempt to neutralize virus particles and how CoV-2 can block that effort. We explain some of the virus's surprising abilities, such as its capacity to ...
- Vaccination from the Misinformation Viruson August 3, 2021 at 5:22 pm
Adjust the colors to reduce glare and give your eyes a break. Use one of the services below to sign in to PBS: You've just tried to add this video to My List. But first, we need you to sign in to ...
- Genetics of the Influenza Viruson September 9, 2019 at 1:44 pm
Reid, A. H., & Tautenberger, J. K. The origin of the 1918 pandemic influenza virus: A continuing enigma. Journal of General Virology 84, 2285–2292 (2003) ...
- Mac Virus Scan: How to protect your Mac from malwareon May 27, 2015 at 11:56 am
Initiating a virus scan is an easy process that usually begins with the user clicking a Scan or Smart Scan button. Expect the scan to take a while if there is a lot of data on your Mac.
via Bing News