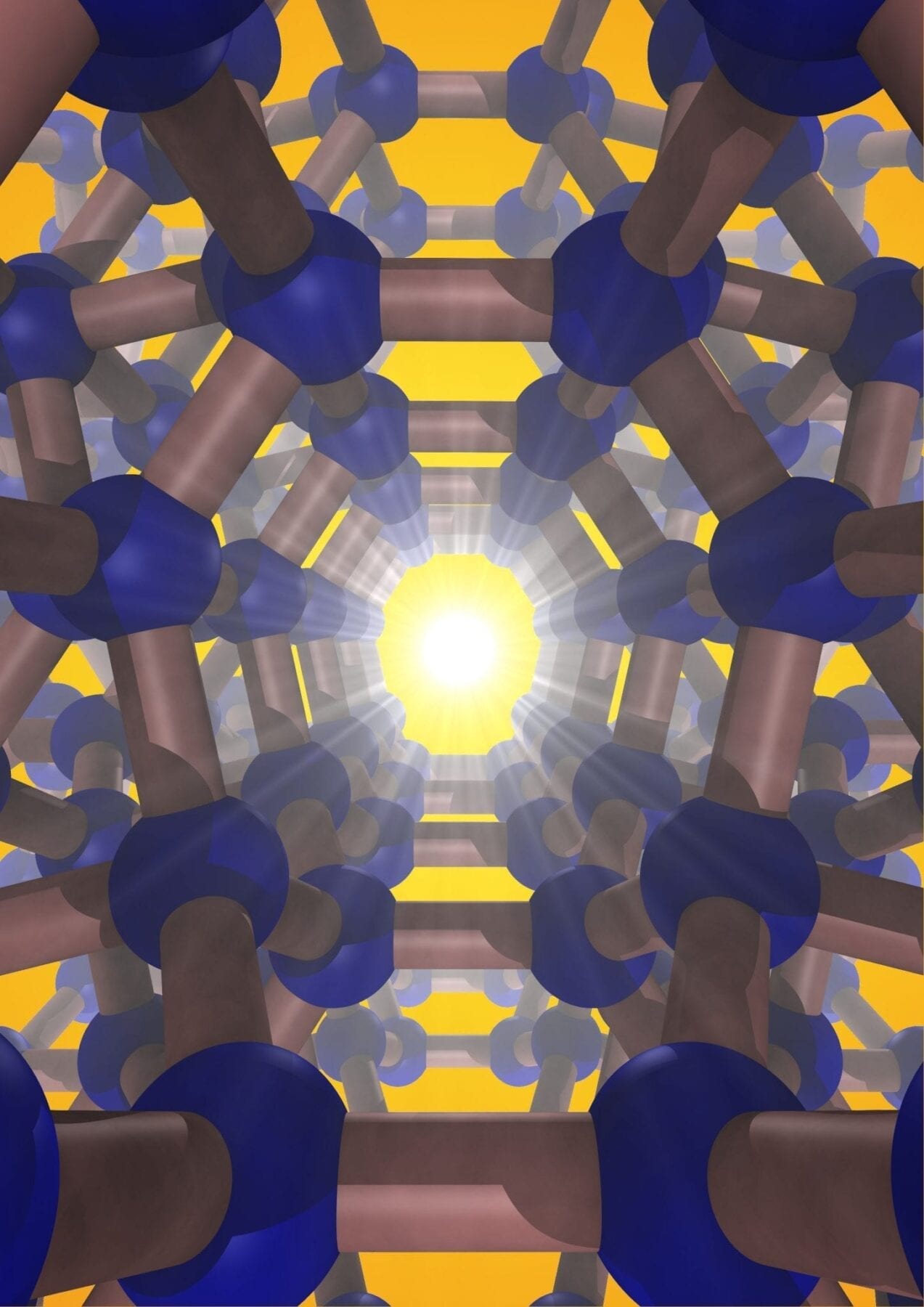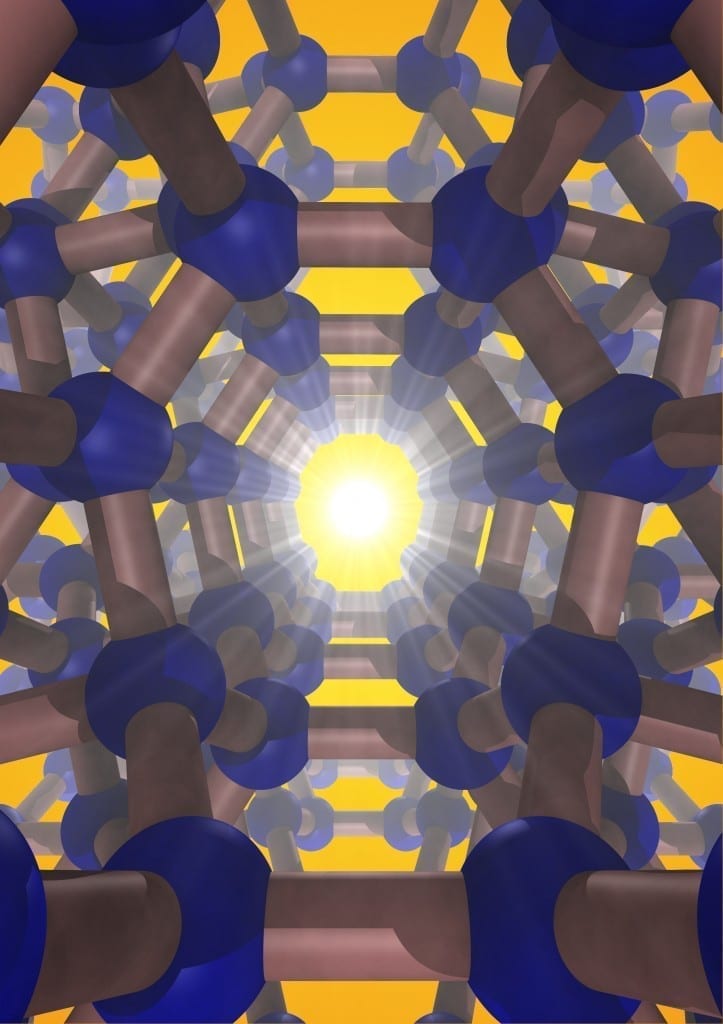
A team of Carnegie scientists led by Timothy Strobel has synthesized an entirely new form of silicon, one that promises even greater future applications.
Silicon is the second most-abundant element in the earth’s crust. When purified, it takes on a diamond structure, which is essential to modern electronic devices—carbon is to biology as silicon is to technology. A team of Carnegie scientists led by Timothy Strobel has synthesized an entirely new form of silicon, one that promises even greater future applications. Their work is published in Nature Materials.
Although silicon is incredibly common in today’s technology, its so-called indirect band gap semiconducting properties prevent it from being considered for next-generation, high-efficiency applications such as light-emitting diodes, higher-performance transistors and certain photovoltaic devices.
Metallic substances conduct electrical current easily, whereas insulating (non-metallic) materials conduct no current at all. Semiconducting materials exhibit mid-range electrical conductivity. When semiconducting materials are subjected to an input of a specific energy, bound electrons can move to higher-energy, conducting states. The specific energy required to make this jump to the conducting state is defined as the “band gap.” While direct band gap materials can effectively absorb and emit light, indirect band gap materials, like diamond-structured silicon, cannot.
In order for silicon to be more attractive for use in new technology, its indirect band gap needed to be altered. Strobel and his team—Carnegie’s Duck Young Kim, Stevce Stefanoski and Oleksandr Kurakevych (now at Sorbonne) —were able to synthesize a new form of silicon with a quasi-direct band gap that falls within the desired range for solar absorption, something that has never before been achieved.
The silicon they created is a so-called allotrope, which means a different physical form of the same element, in the same way that diamonds and graphite are both forms of carbon. Unlike the conventional diamond structure, this new silicon allotrope consists of an interesting open framework, called a zeolite-type structure, which is comprised of channels with five-, six- and eight-membered silicon rings.
The Latest on: High-pressure precursor synthesis
[google_news title=”” keyword=”High-pressure precursor synthesis” num_posts=”10″ blurb_length=”0″ show_thumb=”left”]
via Google News
The Latest on: High-pressure precursor synthesis
- High-Throughput Apparatus Speeds Up Eco-Friendly Nanomaterial Synthesison May 7, 2024 at 1:15 pm
University of Birmingham researcher developed a new high-throughput device that uses mechanochemical synthesis to create libraries of nanomaterials in a faster and more sustainable way.
- 'Better than graphene' material development may improve implantable technologyon May 6, 2024 at 7:01 am
Move over, graphene. There's a new, improved two-dimensional material in the lab. Borophene, the atomically thin version of boron first synthesized in 2015, is more conductive, thinner, lighter, ...
- Towards novel promising perovskite-type ferroelectric materials: High-pressure synthesis of rubidium niobateon April 24, 2024 at 5:00 pm
"The high-pressure synthesis method has reported a variety of materials with perovskite-type structures, including superconductors and magnets. In this study, our focus was on combining niobates ...
- Making diamonds at ambient pressureon April 24, 2024 at 10:49 am
Researchers have grown diamonds under conditions of 1 atmosphere pressure and at 1025 degrees Celsius using a liquid metal alloy composed of gallium, iron, nickel, and silicon, thus breaking the ...
- Scientists develop novel liquid metal alloy system to synthesize diamond under moderate conditionson April 24, 2024 at 8:09 am
Did you know that 99% of synthetic diamonds are currently produced using high-pressure and high-temperature ... HPHT and chemical vapor deposition synthesis methods. Once formed, the diamond ...
- The 15 Best Nitric Oxide Boosters in 2024on April 18, 2024 at 5:06 am
It also affects immune response, inflammation regulation, and blood pressure maintenance ... an amino acid serving as a nitric oxide precursor. Foods high in L-arginine include nuts, seeds ...
- How to Pick a Home Blood Pressure Monitoron April 15, 2024 at 5:00 pm
A home monitor lets you check it often, which can give your doctor a better idea of your true blood pressure. The best way to know if you have high blood pressure is to measure it several times a ...
- Ceramic Nanoparticles: Versatile Building Blocks for Advanced Materialson March 31, 2024 at 8:00 pm
Hydrothermal synthesis involves the reaction of precursor materials in an aqueous medium under high temperature and pressure conditions. This method allows for the formation of highly crystalline ...
- What Are the Symptoms of Scleroderma (Systemic Sclerosis)?on December 20, 2023 at 5:18 pm
Due to the excessive synthesis of collagen, the skin may become tight ... Systemic sclerosis can impact the kidneys in some circumstances, resulting in renal problems. High blood pressure, ...
- Chemical vapor deposition (CVD) and its role in semiconductor fabricationon May 3, 2023 at 2:02 am
Chemical vapor deposition also is a promising approach for scalable synthesis ... pressure, and precursor concentration, as well as the conformal nature of the deposition process. CVD is capable of ...
via Bing News